2.6: Flame test
- Page ID
- 369380
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A flame test is a complex phenomenon that is not fully explained. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal chlorides is injected into a flame, some of the metal ions may gain electrons and become neutral metal atoms. Electrons in the atom can be promoted from the ground state to a higher energy excited state by the strong heat of the flame. The excited electrons ultimately return to the ground state either in one go or in several steps by jumping to lower allowed energy states. When the excited electrons jump from higher to lower allowed energy states they emit electromagnetic radiation of a specific wavelength corresponding to the energy gap between the energy states. Some of these radiations may fall in the visible part of the electromagnetic radiation spectrum. The color we see is a combination of all the colors in the emission spectrum, as illustrated in Fig. 2.7.1.Figure \(\PageIndex{1}\).



The exact gap between the energy levels allowed for electrons varies from one metal to another metal. Therefore, different metals have different patterns of spectral lines in their emission spectrum, and if some of these spectral lines fall in the visible spectrum range, they impart different colors to the flame. For example, the ground-state electron configuration of the sodium atom is 1s22s22p6. When the sodium atom is in the hot flame some of the electrons can jump to any of the higher energy allowed stages, such as 3s, 3p, etc. The familiar intense yellow flame of sodium is a result of excited electrons jumping back from 3p1 to ground state 3s1 level.



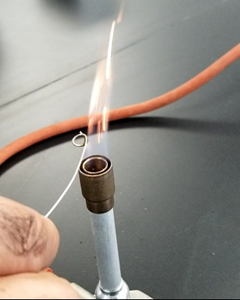
Often metal chloride salts are used for the flame tests as they are water-soluble and easier to vaporize in flame from the solution. Metal chloride salts are first dissolved in water. Other metal salts are first treated with 6M \(\ce{HCl}\) to dissolve them as metal chlorides and then used for the flame test. An inert platinum wire is dipped in the test solution. Usually, the wire has a small loop a the end to make a film of the solution that evaporates in the flame. Air and fuel supply to the flame are adjusted to produce a non-luminous flame. The wire carrying the salt solution is touched on the outer edge of a flame somewhere in the middle of the vertical axis of the flame and the color imparted to the flame is observed. Nichrome wire is a cheaper alternative to platinum wire, though nichrome may slightly alter the flame color. A wooden splint or wooden cotton-tipped applicator are other cheaper alternatives. The wooden splint or cotton swab applicator is first dipped in deionized or distilled water overnight so that the cotton or wood may not burn when placed in a flame for a short time. The salt solution is then applied to the wooden splint end or to the cotton swab and exposed to the flame.
Wooden splint and cotton-tipped applicators are disposable, i.e., they are discarded after one flame test. Platinum wire can be reused after washing. The wire is dipped in 6M \(\ce{HCl}\) and then heated in a flame to red-hot. The process is repeated till the wire does not alter the color of the flame. Then it can be re-used. Nichrome wire can be washed the same way. However, an easier alternative is to cut out the loop of the wire and make a new loop on the fresh end portion. Then use the wire for the next flame test.
Figure \(\PageIndex{2}\) shows that flame tests tested using calcium chloride work equally well with nichrome wire, cotton-tipped applicator, and wooden splint. Figure \(\PageIndex{3}\) shows flam colors of some metal chloride salt solutions exposed to the flame on a cotton swab applicator.










