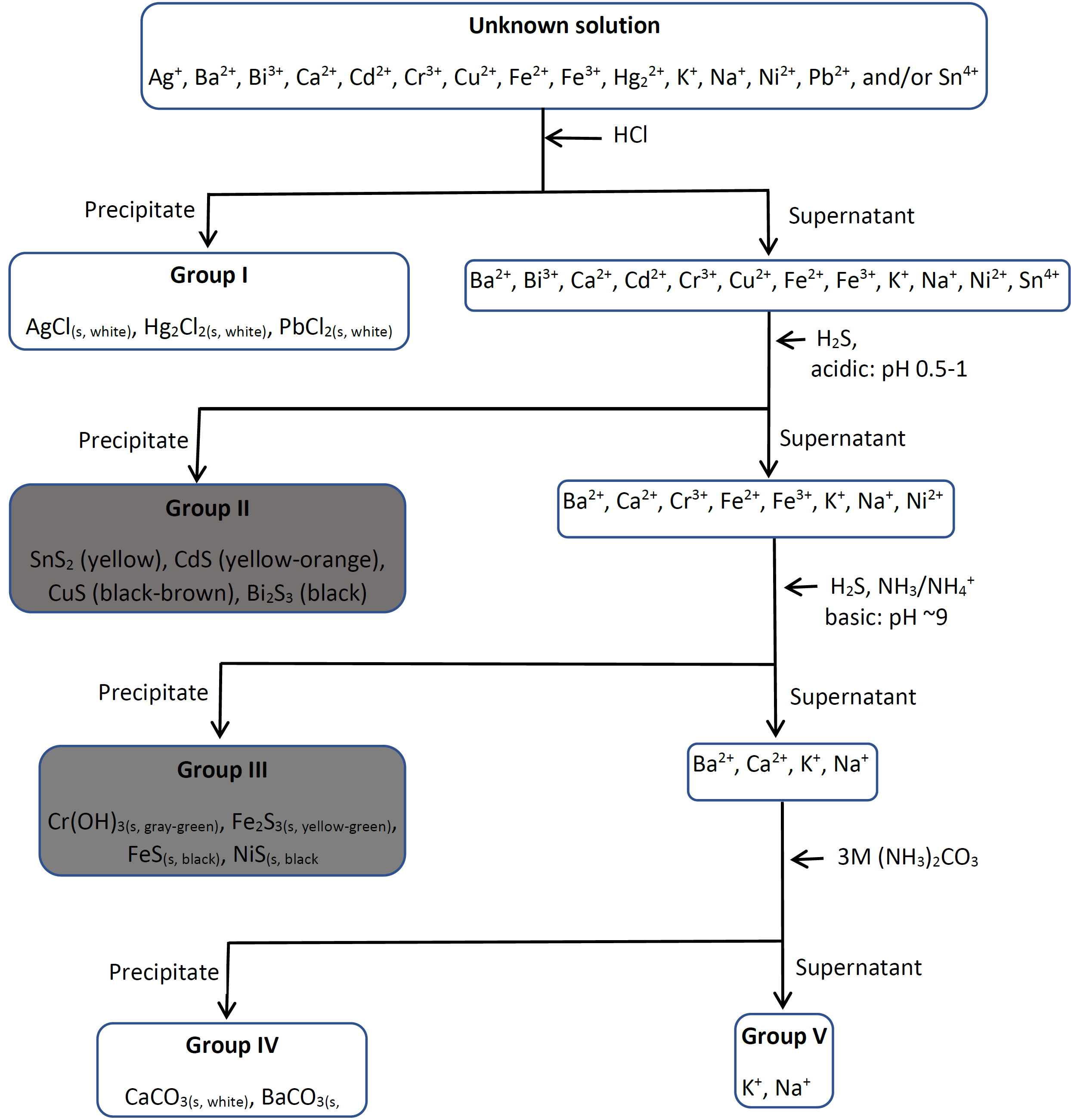
1.5: Separation of cations in groups
- Page ID
- 369481
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Qualitative analysis of cations commonly found in water solution is usually done in three stages:
- ions are separated into broader groups by selective precipitation based on their solubility properties,
- member ions in a group are separated usually by selective dissolution of the precipitates, and
- individual ions are identified by a specific confirmation test.
For the 1st stage, i.e., separation of cations in groups, a suitable reagent is selected that selectively precipitates certain ions leaving the rest of the ions in the solution.
A suitable reagent is the one that:
- almost completely removes the ions belonging to the group so that the residual ions may not interfere in the analysis of the other ions left in the solution,
- should not precipitate out a fraction of ions that do not belong to the group being separated, and
- should not leave behind counter ion that does not interfere in the analysis of rest of the ions.
The reagents are added in an order such that the most selective reagent, the one that precipitates out the least number of ions, is added first.
The fourteen common cations found in water that are selected in these exercises are separated into five groups.
Group I comprises lead II (\(\ce{Pb^{2+}}\)), mercury (I) (\(\ce{Hg2^{2+}}\)), and silver (I) (\(\ce{Ag^{+}}\)) that are selectively precipitated as chlorides by adding 6M \(\ce{HCl}\) to the mixture.
\[\ce{HCl + H2O(l) -> H3O^{+}(aq) + Cl^{-}(aq)}\nonumber\]
\(\ce{HCl}\) solution is selected as a reagent for group I based on the facts: i) it is a source of chloride (\(\ce{Cl^{-}}\)) ion which is the most selective reagent that makes insoluble salts with only \(\ce{Pb^{2+}}\), \(\ce{Hg2^{2+}}\), and \(\ce{Ag^{+}}\) (recall soluble ions rule#3 described in section 1.1), ii) it leaves behind \(\ce{H3O^{+}}\) that makes the solution acidic which is beneficial for separation of cations of the next group.
Group II comprises tin(IV) (\(\ce{Sn^{4+}}\)), cadmium(II) (\(\ce{Cd^{2+}}\)), copper(II) (\(\ce{Cu^{2+}}\)), and bismuth(III) (\(\ce{Bi^{3+}}\)) that are selectively precipitated as sulfides by adding \(\ce{H2S}\) reagent in an acidic medium. \(\ce{H2S}\) is a source of sulfide (\(\ce{S^{2-}}\)) ion in water:
\[\ce{H2S(aq) + 2H2O(l) -> 2H3O^{+}(aq) + S^{2-}(aq)}\nonumber\]
The \(\ce{S^{2-}}\) ion makes insoluble salts with many cations as stated by insoluble ions rule#1 in section 1.1, i.e., “Hydroxide (\(\ce{OH^{-}}\)) and sulfides (\(\ce{S^{2-}}\)) are insoluble except when the cation is a heavy alkaline earth metal ion: \(\ce{Ca^{2+}}\), \(\ce{Ba^{2+}}\), and \(\ce{Sr^{2+}}\), or an alkali metal ion, or ammonia.”
\(\ce{H2S}\) in acidic medium is selected as a source of \(\ce{S^{2-}}\) which is the reagent for selective precipitation of group II, because the concentration of \(\ce{S^{2-}}\) can be controlled by adjusting pH. Acidic medium has higher [\(\ce{H3O^{+}}\)] that decreases \(\ce{S^{2-}}\) due to the common ion effect of \(\ce{HeO{+}}\) ion. Therefore, among the sulfide insoluble salts, only the group II cations having very low solubility are selectively precipitated.
Group III comprises chromium(III) (\(\ce{Cr^{3+}}\)), iron(II) (\(\ce{Fe^{2+}}\)), iron(III) (\(\ce{Fe^{3+}}\)), and nickel(II) (\(\ce{Ni^{2+}}\)) selectively precipitated by as insoluble hydroxides and sulfides by adding \(\ce{H2S}\) in alkaline medium with pH maintained at ~9 by \(\ce{NH3}\)/\(\ce{NH4^{+}}\) buffer.
\(\ce{H2S}\) in an alkaline medium is the reagent for the selective precipitation of group III cations.
When pH is set at 9 by \(\ce{NH3}\)/\(\ce{NH4^{+}}\) buffer, \(\ce{OH^{-}}\) concentration is high enough to precipitate group III cations as insoluble hydroxide except for nickel that forms soluble coordination complex ion with ammonia. When \(\ce{H2S}\) is added in an alkaline medium, it produces a higher concentration of \(\ce{S^{2-}}\) due to the removal of \(\ce{H3O^{+}}\) from its equilibrium by reacting with \(\ce{OH^{-}}\):
\[\ce{H3O^{+}(aq) + OH^{-}(aq)-> 2H2O(l)} \nonumber\]
All of the group III cations are converted to insoluble sulfides except chromium.
Group IV comprise of calcium (\(\ce{Ca^{2+}}\)) and barium (\(\ce{Ba^{2+}}\)) selectively precipitate as insoluble carbonates by adding ammonium carbonate (\(\ce{(NH4)2CO3}\)) as a source of carbonate (\(\ce{CO3^{2-}}\)) ion:
\[\ce{(NH4)2CO3(s) + 2H2O <=> 2NH4^{+}(aq) + CO3^{2-}(aq)}\nonumber\]
The \(\ce{CO3^{2-}}\) ion makes insoluble salts with many cations as stated by insoluble ions rule#2 in section 1.1, i.e., “Carbonates (\(\ce{CO3^{2-}}\)), phosphates (\(\ce{PO4^{3-}}\)), and oxide (\(\ce{O^{2-}}\)) are insoluble except when the cation is an alkali metal ion or ammonia.” All other ions have already been precipitated at this stage in groups I, II, and III except group IV cations and alkali metal ions.
The \(\ce{CO3^{2-}}\) ion is a selective reagent for group IV cations because
Group V comprises alkali metal ions, i.e., sodium (\(\ce{Na^{+}}\)) and potassium (\(\ce{K^{+}}\)) in the mixture of ions selected. According to soluble ions rule#1 in section 1.1, alkali metal and ammonium ions form soluble salts. So, group V cations remain in solution after groups I, II, III, and IV cations are removed as insoluble, chloride, sulfide in acid medium, sulfide in basic medium, and carbonates, respectively.
The separation of cations in groups, along with the separation of ions within a group and their confirmation tests are described in detail in later chapters. The flow chart shown below shows the summary of the separation of common cations in water into the five groups.