6.3C: Spectral Interferences
- Page ID
- 111882
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Particulate matter in a flame will scatter light from the hollow cathode lamp. Some metals are prone to forming solid refractory oxides in the flame that scatter radiation. Organic matter in a flame may lead to carbonaceous particles that scatter radiation. This is a problem since the detector cannot distinguish the difference between light that is scattered and light that is absorbed.
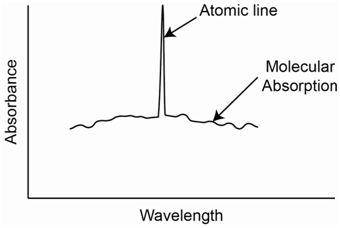
Similarly, molecular species in a flame exhibit broadband absorption of light. Figure \(\PageIndex{13}\) shows a plot of an atomic absorption line superimposed over molecular absorption. As with scattered radiation, the detector cannot distinguish broadband absorption from molecular species from line absorption by atomic species.
Can you design a feature that could be incorporated into an atomic absorption spectrophotometer than can account for both scattered light and light absorbed by molecular species?
To address this question, we need to think back to the previous discussion of the source requirement for atomic absorption spectrophotometers. Earlier we saw that it was not possible to use a continuum source with a monochromator since the atomic absorption was so negligible as to be non-detectable. However, a continuum source will measure molecular absorption and will respond to any scattered radiation. The answer is to alternately send the output from the hollow cathode lamp and a continuum source (the common one used in AA instruments is a deuterium lamp) to the flame. The output of the hollow cathode lamp will be diminished by atomic absorption, molecular absorption and scatter. The continuum lamp will only be diminished by molecular absorption and scatter, since any contribution from atomic absorption is negligible. By comparing these, it is possible to correct the signal measured when the hollow cathode lamp passes through the flame for scattered radiation and molecular absorption. In atomic absorption spectroscopy, this process is referred to as background correction.
An alternative way of getting a broadened source signal to pass through the flame is known as the Smith-Hieftje method (named after the investigators who devised this method). The Smith-Hieftje method only uses a hollow cathode lamp. Earlier, when we discussed hollow cathode lamps, we learned that the argon pressure inside the lamp was kept low to avoid collisional broadening. We also learned that the current was not set to a high value because it would sputter off too many atoms and shorten the lamp lifetime. Another observation when running a hollow cathode lamp at a high current is that the lamp emission lines broaden. This occurs because, at a high current, so many atoms get sputtered off into the hollow cathode that they collide with each other and broaden the wavelength distribution of the emitted light. The Smith-Hieftje method relies on using a pulsed lamp current. For most of the time, the lamp is run at its optimal current and emits narrow lines that would diminish when passing through the flame due to atomic absorption, molecular absorption and scatter. For a brief pulse of time, the current is set to a very high value such that the lamp emits a broadened signal. When this broadened signal passes through the flame, atomic absorption is negligible and only molecular absorption and scatter decrease the intensity of the beam.
A third strategy is to use what is known as the “two-line” method. This can be used in a situation where you have a source that emits two narrow atomic lines, one of which is your analysis wavelength and the other of which is close by. Looking back at Figure \(\PageIndex{13}\), the analysis wavelength is diminished in intensity by atomic absorption, molecular absorption and scattering. A close by line does not have any atomic absorption and only is reduced in intensity by molecular absorption and scattering. While it might at first seem difficult to see how it is possible to get nearby atomic lines for many elements, there is something known as the Zeeman Effect that can be used for this purpose. Without going into the details of the Zeeman Effect, what is important to know is that exposing an atomic vapor to a strong magnetic field causes a slight splitting of the energy levels of the atom causing a series of closely spaced lines for each electronic transition. The neighboring lines are about 0.01 nm from each other, making them ideal for monitoring background molecular absorption and scatter. Corrections using the Zeeman Effect are more reliable than those using a continuum source. The magnetic field can be applied either to the hollow cathode lamp or the atomization source. The method is useful in flame and graphite furnace measurements.


