1.3A: Radiation Sources
- Page ID
- 111326
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Describe the desirable features of a radiation source for a spectrophotometer.
An obvious feature is that the source must cover the region of the spectrum that is being monitored. Beyond that, one important feature is that the source has high power or intensity, meaning that it gives off more photons. Since any detector senses signal above some noise, having more signal increases what is known as the signal-to-noise ratio and improves the detection limit. The second important feature is that the source be stable. Instability on the power output from a source can contribute to noise and can contribute to inaccuracy in the readings between standards and unknown samples.
Plot the relative intensity of light emitted from an incandescent light bulb (y-axis) as a function of wavelength (x-axis). This plot is a classic observation known as blackbody radiation. On the same graph, show the output from a radiation source that operated at a hotter temperature.
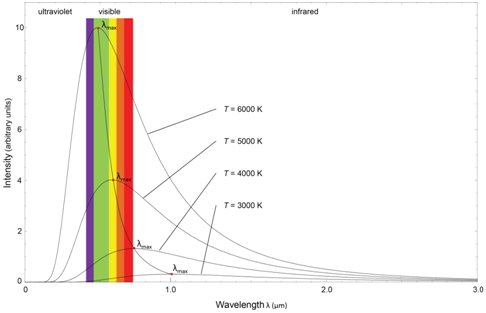
As shown in Figure \(\PageIndex{7}\), the emission from a blackbody radiator has a specific wavelength that exhibits maximum intensity or power. The intensity diminishes at shorter and longer wavelengths. The output from a blackbody radiator is a function of temperature. As seen in Figure \(\PageIndex{7}\), at hotter temperatures, the wavelength with maximum intensity moves toward the ultraviolet region of the spectrum.
Examining the plots in Figure \(\PageIndex{7}\), what does this suggest about the power that exists in radiation sources for the infrared portion of the spectrum?
The intensity of radiation in the infrared portion of the spectrum diminishes considerably for most blackbody radiators, especially at the far infrared portions of the spectrum. That means that infrared sources do not have high power, which ultimately has an influence on the detection limit when using infrared absorption for quantitative analysis.
Blackbody radiators are known as continuous sources. An examination of the plots in Figure \(\PageIndex{7}\) shows that a blackbody radiator emits radiation over a large continuous band of wavelengths. A monochromator can then be used to select out a single wavelength needed for the quantitative analysis. Alternatively, it is possible to scan through the wavelengths of radiation from a blackbody radiator and record the spectrum for the species under study.
Explain the advantages of a dual- versus single-beam spectrophotometer.
One way to set up a dual-beam spectrophotometer is to split the beam of radiation from the source and send half through a sample cell and half through a reference cell. The reference cell has a blank solution in it. The detector is set up to compare the two signals. Instability in the source output will show up equally in the sample and reference beam and can therefore be accounted for in the measurement. Remember that the intensity of radiation from the source varies with wavelength and drops off toward the high and low energy region of the spectrum. The changes in relative intensity can be accounted for in a dual-beam configuration.
A laser (LASER = Light Amplification by Stimulated Emission of Radiation) is a monochromatic source of radiation that emits one specific frequency or wavelength of radiation. Because lasers put out a specific frequency of radiation, they cannot be used as a source to obtain an absorbance spectrum. However, lasers are important sources for many spectroscopic techniques, as will be seen at different points as we further develop the various spectroscopic methods. What you probably know about lasers is that they are often high-powered radiation sources. They emit a highly focused and coherent beam. Coherency refers to the observation that the photons emitted by a laser have identical frequencies and waves that are in phase with each other.
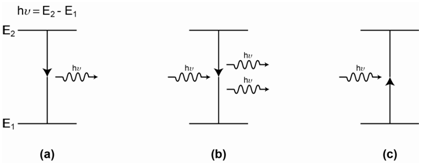
A laser relies on two important processes. The first is the formation of a population inversion. A population inversion occurs for an energy transition when more of the species are in the excited state than are in the ground state. The second is the process of stimulated emission. Emission is when an excited state species emits radiation (Figure \(\PageIndex{8}\)a). Absorption occurs when a photon with the exact same energy as the difference in energy between the ground and excited state of a species interacts with and transfers its energy to the species to promote it to the excited state (Figure \(\PageIndex{8}\)c). Stimulated emission occurs when an incident photon that has exactly the same energy as the difference in energy between the ground and excited state of a transition interacts with the species in the excited state. In this case, the extra energy that the species has is converted to a photon that is emitted. In addition, though, the incident photon also is emitted. One final point is that the two photons in the stimulated emission process have their waves in phase with each other (are coherent) (Figure \(\PageIndex{8}\)b). In absorption, one incident photon comes in and no photons come out. In stimulated emission, one incident photon comes in and two photons come out.
Why is it impossible to create a 2-level laser?
A 2-level laser involves a process with only two energy states, the ground and excited state. In a resting state, the system will have a large population of species in the ground state (essentially 100% as seen in Figure \(\PageIndex{9}\)) and only a few or none in the excited state. Incident radiation of an energy that matches the transition is then applied and ground state species absorb photons and become excited. The general transition process is illustrated in Figure \(\PageIndex{9}\)a.
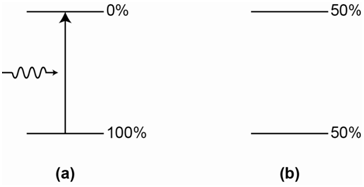
Species in the excited state will give up the excess energy either as an emitted photon or heat to the surroundings. We will discuss this in more detail later on, but for now, it is acceptable to realize that excited state species have a finite lifetime before they lose their energy and return to the ground state. Without worrying about the excited state lifetime, let’s assume that the excited species remain in that state and incident photons can continue to excite additional ground state species into the excited state. As this occurs, the number of species in the excited state (e.g., the excited state population) will grow and the number in the ground state will diminish. The key point to consider is the system where 50% of the species are in the excited state and 50% of the species are in the ground state, as shown in Figure \(\PageIndex{9}\)b.
For a system with exactly equal populations of the ground and excited state, incident photons from the radiation source have an equal probability of interacting with a species in the ground or excited state.
If a photon interacts with a species in the ground state, absorption of the photon occurs and the species becomes excited. However, if another photon interacts with a species in the excited state, stimulated emission occurs, the species returns to the ground state and two photons are emitted. The net result is that for every ground state species that absorbs a photon and becomes excited there is a corresponding excited species that undergoes stimulated emission and returns to the ground state. Therefore it is not possible to get beyond the point of a 50-50 population and never possible to get a population inversion. A 2-level system with a 50-50 population is said to be a saturated transition.
Using your understanding of a 2-level system, explain what is meant by a 3-level and 4-level system. 3- and 4-level systems can function as a laser. How is it possible to achieve a population inversion in a 3- and 4-level system?
The diagrams for a 3-level and 4-level laser system are shown in Figures 1.10 and 1.11, respectively.
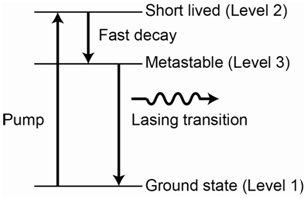
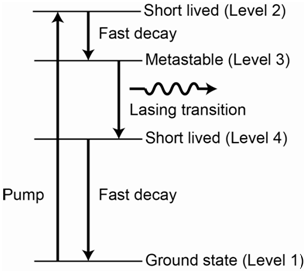
There are certain important features that are necessary to have something function as a 3- or 4-level laser. One is that there has to be a favorable relaxation process in which the species converts or transitions between the second and third levels in the diagrams. The transition from level 2 to level 3 must be more favorable than a transition from level 2 to level 1. Another relates to the relative lifetimes of the excited state levels. It must be the case that the lifetime of the species in level 3 is longer than the lifetime of the species in level 2.
Assuming the two features described above are met, it is now possible to excite species from level 1 to level 2 using the radiation source. Species then transition to level 3 but, because of the longer lifetime, are effectively “stuck” there before returning back to the ground state (level 1). For the 3-level system, If they are stuck in level 3 long enough, it may be possible to deplete enough of the population from level 1 such that the population in level 3 is now higher than the population in level 1. The level 3 to level 1 transition is the lasing transition and note that the incident photons from the source have a different energy than this transition so no stimulated emission occurs. When the population inversion is achieved, a photon emitted from a species in level 3 can interact with another species that is excited to level 3, causing the stimulated emission of two photons. These emitted photons can interact with additional excited state species in level 3 to cause more stimulated emission and the result is a cascade of stimulated emission. This large cascade or pulse of photons all have the same frequency and are coherent. The process of populating level 3 in either the 3- or 4-level system using energy from the incident photons from the radiation source is referred to as optical pumping.
For the 4-level laser, the lasing transition is from level 3 to level 4, meaning that a population inversion is needed between levels 3 and 4 and not levels 3 and 1.
Which of the two (3- or 4-level system) is generally preferred in a laser and why?
Since the population of level 4 is much lower than the population of level 1, it is much easier to achieve a population inversion in a 4-level laser compared to a 3-level laser. Therefore, the 4-level laser is generally preferred and more common than a 3-level laser.


