31.1: Thermogravimetry
- Page ID
- 363138
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One method for determining the products of a thermal decomposition is to monitor the sample’s mass as a function of temperature, a process called a thermogravimetric analysis (TGA) or thermogravimetry. Figure 31.1.1 shows a typical thermogram in which each change in mass—each “step” in the thermogram—represents the loss of a volatile product. As the following example illustrates, we can use a thermogram to identify a compound’s decomposition reactions.
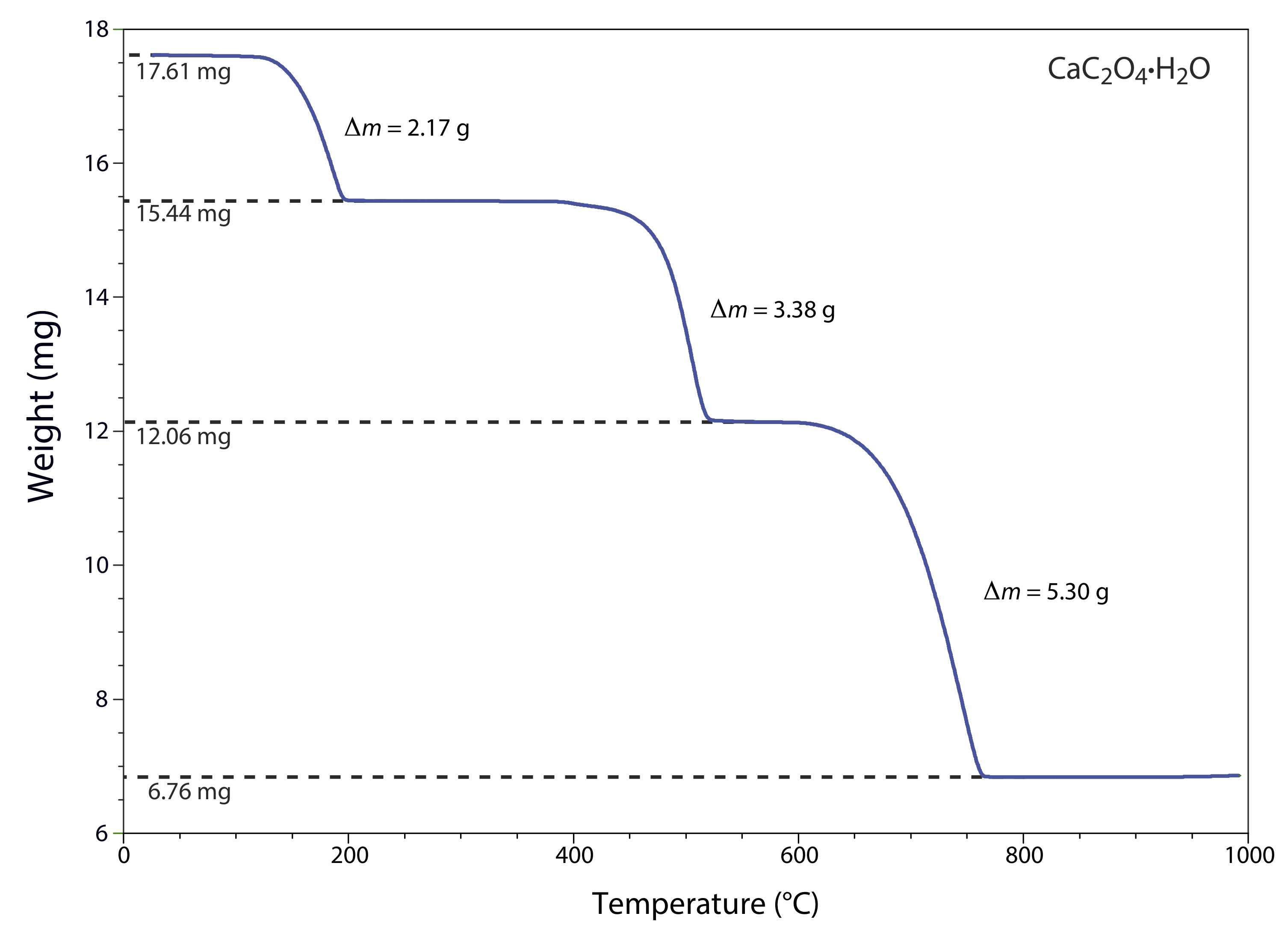
The thermogram in Figure 31.1.1 shows the mass of a sample of calcium oxalate monohydrate, CaC2O4•H2O, as a function of temperature. The original sample of 17.61 mg was heated from room temperature to 1000oC at a rate of 20oC per minute. For each step in the thermogram, identify the volatilization product and the solid residue that remains.
Solution
From 100–250oC the sample loses 17.61 mg – 15.44 mg, or 2.17 mg, which is
\[\frac{2.17 \ \mathrm{mg}}{17.61 \ \mathrm{mg}} \times 100=12.3 \% \nonumber \]
of the sample’s original mass. In terms of CaC2O4•H2O, this corresponds to a decrease in the molar mass of
\[0.123 \times 146.11 \ \mathrm{g} / \mathrm{mol}=18.0 \ \mathrm{g} / \mathrm{mol} \nonumber \]
The product’s molar mass and the temperature range for the decomposition, suggest that this is a loss of H2O(g), leaving a residue of CaC2O4.
The loss of 3.38 mg from 350–550oC is a 19.2% decrease in the sample’s original mass, or a decrease in the molar mass of
\[0.192 \times 146.11 \ \mathrm{g} / \mathrm{mol}=28.1 \ \mathrm{g} / \mathrm{mol} \nonumber \]
which is consistent with the loss of CO(g) and a residue of CaCO3.
Finally, the loss of 5.30 mg from 600-800oC is a 30.1% decrease in the sample’s original mass, or a decrease in molar mass of
\[0.301 \times 146.11 \ \mathrm{g} / \mathrm{mol}=44.0 \ \mathrm{g} / \mathrm{mol} \nonumber \]
This loss in molar mass is consistent with the release of CO2(g), leaving a final residue of CaO. The three decomposition reactions are
\[\begin{array}{c}{\mathrm{CaC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}(s) \rightarrow \ \mathrm{CaC}_{2} \mathrm{O}_{4}(s)+2 \mathrm{H}_{2} \mathrm{O}(l)} \\ {\mathrm{CaC}_{2} \mathrm{O}_{4}(s) \rightarrow \ \mathrm{CaCO}_{3}(s)+\mathrm{CO}(g)} \\ {\mathrm{CaCO}_{3}(s) \rightarrow \ \mathrm{CaO}(s)+\mathrm{CO}_{2}(g)}\end{array} \nonumber \]
Identifying the products of a thermal decomposition provides information that we can use to develop an analytical procedure. For example, the thermogram in Figure 31.1.1 shows that we must heat a precipitate of CaC2O4•H2O to a temperature between 250 and 400oC if we wish to isolate and weigh CaC2O4. Alternatively, heating the sample to 1000oC allows us to isolate and weigh CaO.
Under the same conditions as Figure 31.1.1 , the thermogram for a 22.16 mg sample of MgC2O4•H2O shows two steps: a loss of 3.06 mg from 100–250oC and a loss of 12.24 mg from 350–550oC. For each step, identify the volatilization product and the solid residue that remains. Using your results from this exercise and the results from Example 31.1.1 , explain how you can use thermogravimetry to analyze a mixture that contains CaC2O4•H2O and MgC2O4•H2O. You may assume that other components in the sample are inert and thermally stable below 1000oC.
- Answer
-
From 100–250oC the sample loses 13.8% of its mass, or a loss of
\[0.138 \times 130.34 \ \mathrm{g} / \mathrm{mol}=18.0 \ \mathrm{g} / \mathrm{mol} \nonumber \]
which is consistent with the loss of H2O(g) and a residue of MgC2O4.
From 350–550oC the sample loses 55.23% of its original mass, or a loss of
\[0.5523 \times 130.34 \ \mathrm{g} / \mathrm{mol}=71.99 \ \mathrm{g} / \mathrm{mol} \nonumber \]
This weight loss is consistent with the simultaneous loss of CO(g) and CO2(g), leaving a residue of MgO.
We can analyze the mixture by heating a portion of the sample to 300oC, 600oC, and 1000oC, recording the mass at each temperature. The loss of mass between 600oC and 1000oC, \(\Delta m_2\), is due to the loss of CO2(g) from the decomposition of CaCO3 to CaO, and is proportional to the mass of CaC2O4•H2O in the sample.
\[\mathrm{g} \ \mathrm{CaC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}=\Delta m_{2} \times \frac{1 \ \mathrm{mol} \ \mathrm{CO}_{2}}{44.01 \ \mathrm{g} \ \mathrm{CO}_{2}} \times \frac{146.11 \ \mathrm{g} \ \mathrm{CaC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}}{\mathrm{mol} \ \mathrm{CO}_{2}} \nonumber \]
The change in mass between 300oC and 600oC, \(\Delta m_1\), is due to the loss of CO(g) from CaC2O4•H2O and the loss of CO(g) and CO2(g) from MgC2O4•H2O. Because we already know the amount of CaC2O4•H2O in the sample, we can calculate its contribution to \(\Delta m_1\).
\[\left(\Delta m_{1}\right)_{\mathrm{Ca}}=\mathrm{g} \ \mathrm{CaC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}=\Delta m_{2} \times \frac{1 \ \mathrm{mol} \ \mathrm{CO}}{146.11 \ \mathrm{g} \ \mathrm{CaC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}} \times \frac{28.01 \ \mathrm{g} \ \mathrm{CO}}{\mathrm{mol} \ \mathrm{CO}} \nonumber \]
The change in mass between 300oC and 600oC due to the decomposition of MgC2O4•H2O
\[\left(m_{1}\right)_{\mathrm{Mg}}=\Delta m_{1}-\left(\Delta m_{1}\right)_{\mathrm{Ca}} \nonumber \]
provides the mass of MgC2O4•H2O in the sample.
\[\mathrm{g} \ \mathrm{MgC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}=\left(\Delta m_{1}\right)_{\mathrm{Mg}} \times \frac{1 \ \mathrm{mol}\left(\mathrm{CO} \ + \ \mathrm{CO}_{2}\right)}{130.35 \ \mathrm{g} \ \mathrm{MgC}_{2} \mathrm{O}_{4} \cdot \mathrm{H}_{2} \mathrm{O}} \times \frac{78.02 \ \mathrm{g} \ \left(\mathrm{CO} \ + \ \mathrm{CO}_{2}\right)}{\mathrm{mol}\ \left(\mathrm{CO} \ + \ \mathrm{CO}_{2}\right)} \nonumber \]
Instrumentation
In a thermogravimetric analysis, the sample is placed on a small balance pan attached to one arm of an electromagnetic balance (Figure 31.1.2 ). The sample is lowered into an electric furnace and the furnace’s temperature is increased at a fixed rate of a few degrees per minute while monitoring continuously the sample’s weight. The instrument usually includes a gas line for purging the volatile decomposition products out of the furnace, and a heat exchanger to dissipate the heat emitted by the furnace.

Applications
Perhaps the most important application gravimetry is exploring a compound's thermal stability, as illustrated in Figure \(\PageIndex{1}\) and Exercise \(\PageIndex{1}\) for calcium oxalate hydrate. TGA is particularly useful for studying the thermal stability of polymers.


