Geometry and Isomerism in Coordination Compounds (Worksheet)
- Page ID
- 127050
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
Learning Objectives
- Identify various types of isomers
- Predict the types of isomers possible from molecular formulas of coordination compounds
- Identify the geometry of a compound from the numbers and types of isomers
Coordination compounds can form different isomers. Two main types of isomerism exhibited by coordination compounds are stereoisomerism and structural isomerism. Stereoisomers include both geometric and optical isomers. Structural isomers include linkage, ionization, coordination, and hydrate isomers. The reactivities and properties of different isomers are often not the same and contribute to the wonderful array of applications of coordination compounds.
Stereoisomerism
Model 1: A square planar complex with the formula [MA2B2] can form two distinct isomers, a cis and a trans (where M represents a metal ion or atom and A and B are ligands).
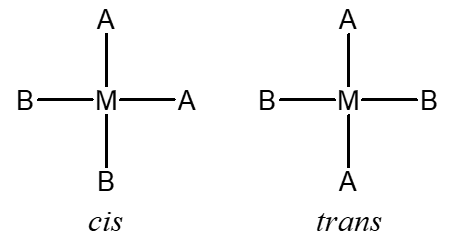
Critical Thinking Questions
- Similar to the case described in Model 1, a square-planar complex with the formula [MA2BC] can form a cis and a trans isomer. Draw and label the two possible isomers.
- A square-planar complex with formula [MABCD] can form three isomers. These isomers are called “A trans to B” and so on. Draw and label the three possible isomers.
- A tetrahedral complex with the formula [MABCD] can form two enantiomers. Draw the two possible structures. (Hint: think back to organic chemistry!)
- Cis and trans isomers are possible for octahedral complexes with the formula [MA4B2]. Draw and label the two possible isomers.
- Two isomers are possible for octahedral complexes with the formula [MA3B3]. In one isomer, the three A ligands are arranged on one plane with the three B ligands in a perpendicular plane; this configuration is called the meridional or mer isomer. In the other isomer, the three A ligands are adjacent and occupy the corners of one triangular face (as do the B ligands); this configuration is called the facial or fac isomer. Draw and label the two possible isomers for an octahedral complex with the formula [MA3B3].
- Five isomers are possible for an octahedral complex with the formula [MA2B2C2]. Draw and label the possible isomers (don’t forget to consider enantiomeric pairs!).
- Use your answers from CTQ 1-6 to complete the summary table below.
| Geometry | Names | Number of isomers | Number of enantiomeric pairs |
|---|---|---|---|
| Coordination Number=4 | |||
| Square-planar | |||
| [MA2B2] |
|
2 | n/a |
| [MA2BC] |
|
2 | n/a |
| [MABCD] | |||
| Tetrahedral | |||
| [MABCD] | |||
| Coordination Number=6 | |||
| Octahedral | |||
| [MA3B3] | |||
| [MA2B2C2] | |||
Other types of isomers
Model 2: Linkage isomers are possible for ambidentate ligands such as the thiocyanate ion (NCS-) which can attach via the nitrogen or the sulfur atom or NO2- which can attach via the nitrogen or the oxygen atom.
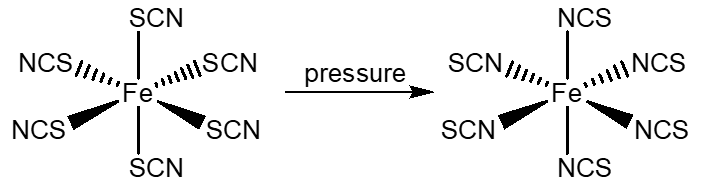
Hexathiocyanato ferrate undergoes isomerization to hexaisothiocyanato ferrate under high pressures. The conversion is essentially complete at 150 kb (Fung and Drickamer, PNAS 1958, 62, 38-43).
Ionization isomers are formed when the ligand and counter ion are exchanged: for example, a compound consisting of Pt(NH3)4 with two Cl- and two Br- could form [Pt(NH3)4Cl2]Br2 or [Pt(NH3)4Br2]Cl2.
Coordination isomers form when different complex ions can form from the same molecular formula: CoCr(NH3)6(CN)6 could produce [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6].
Hydrate isomers are formed when at least one of the ligands in a coordination complex is water. [Co(NH3)4(OH2)Cl]Cl2 and [Co(NH3)4Cl2]Cl·H2O are examples of hydrate isomers.
Critical Thinking Questions
- Identify the type of isomerism possible for the following complexes
- [RuBr(NH3)5]Cl and [RuCl(NH3)5]Br
- [Co(NO2)(NH3)5]2+
- [Cr(OH2)6]Cl3 and [Cr(OH2)5Cl]Cl2
- CrCl3 • 6 H2O has three distinctly different crystalline compounds, A is violet, B is blue-green, and C is dark green. Each compound is octahedral. Upon titration with excess silver nitrate, A produces three equivalents of AgCl, B produces two equivalents of AgCl, and C produces one equivalent of AgCl. Determine the formulas of the compounds. Match the colors to the formulas. What type of isomers are these compounds?
Problems
- Are isomers possible for the following coordination compounds? If so, draw the isomers and label each:
- [IrCl(CO)(PPh3)2]
- [Co(NO2)3(NH3)3] (NO2- linked through the N)
- [CoCl2(NH3)3(OH2)]+
- [CoBrClI(OH2)] (tetrahedral)
- [Fe(CN)2(CH3NC)4]
- How many isomers are possible for the complex [Cr(OH2)3BrClI]? Draw each.
References
- Jens-Uwe Kuhn, Santa Barbara City College
- Jessica Martin, Northeastern State University

