7: Atomic Structure
- Page ID
- 140334
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Atomic Structure
The Nuclear Model
In 1911 Ernest Rutherford interpreted several experiments to form what is
called the Nuclear Model of the Atom.
• Take a pen and drop it 3-4 times from about a foot over the page so it leaves a spot where it hits. Each time you drop your pen you are ‘shooting’ a helium nucleus at gold atoms like Rutherford did in lab experiments.

-
Did any of your pen marks hit the center of an atom?
-
What can you conclude about the relative size of a nucleus in comparison to the entire atom? (Note: This may seem obvious, but scientists were not able to see this like you can.)
-
In experiments similar to the one you performed approximately 1 in 10,000 helium nuclei collided with the center of a gold atom. What does this tell you about the rest of the atom?
-
Utilize the mass data below to comment on the relative sizes of electrons, neutrons and protons.
| Particle | ||
| Electron | Outside the nucleus | 9.109 x 10-31 kg |
| Proton | ||
| Neutron | Nucleus |
1.674 x 10-27 kg |
- Summary: Fill in the blanks in the sentence below:
The majority of the mass of an atom is contained _____________ (in the nucleus, outside the nucleus), which has a very ___________ (low, high) density.
Atomic Composition
Atoms are made of three types of nuclear particles: neutrons, electrons and protons. The ratio of these particles determines the element, the mass and the charge on the atom.

Atomic Composition
-
How many protons are found in 12C, 13C, and 14C?
-
How many neutrons are found in 12C, 13C, and 14C?
-
How many electrons are found in 12C, 13C, and 14C?
-
The atomic number (Z) tells you the number of ___________. Where do you find the atomic number on the periodic table?
-
What subatomic particle differs in isotopes of a particular element?
-
How is the atomic mass (the left-hand superscript in a symbol) determined from the structure of an atom?
-
How many neutrons are in the following isotopes of nickel (Ni)?
a) 58Ni
b) 60Ni
Application Problems:
• Complete the blanks in the table.
| Element | Number of Elections | Number of Protons | Number of Neutrons | Approximate mass in amus |
| 12C | 6 | 6 | 6 | |
| 13C | 6 | 6 | 13 | |
| 14C | ||||
| 16C | 8 | 8 | 16 | |
| 16C2- ion | 10 | 8 | ||
| 18O | 10 | |||
| 23Na | 11 | 11 | 23 | |
| 23Na+ ion | 11 | 12 |
-
What is the approximate mass in atomic mass units of one 1H atom? How about one 12C atom?
-
Define the following terms:
a) mass number
b) atomic number
c) isotope
d) ion
-
Make sketches of the three major isotopes of hydrogen showing the location and quantity of the major subatomic particles. The isotopes are1H, 2H, and 3H.
Isotopes
Most elements exist as mixtures of different isotopes. The number of isotopes varies for each element. The atomic masses on the periodic table are averages of all of the isotopes for that element.
Chlorine contains two major isotopes, 35Cl and 37Cl.
-
Record the mass of Chlorine (Cl) from the periodic table and explain why it cannot contain only one isotope.
Average mass of Cl isotopes: _____________
-
Based on the mass of Cl from the periodic table, which estimate below is correct for the mixture of 35Cl and 37Cl?
a) An equal mixture of 35Cl and 37Cl.
b) About 25% 35Cl and 75% 37Cl.
c) About 75% 35Cl and 25% 37Cl.
d) Almost all 37Cl. -
The element cobalt (Co, Z = 27) is virtually 100% of one isotope. How many neutrons are in the major isotope of cobalt?
-
Carbon contains three isotopes, 12C, 13C, and 14C, based on the periodic table mass what is the major isotope of carbon?
Isotopic Ratios
You have a collection of marbles and determine that 25% of them have a mass of 5.0 g and 75% of them have a mass of 9.0 g. If you had four marbles you could determine the average mass by the following calculation:
$$
\frac { ( 1 \times 5.0 \mathrm { g } ) + ( 3 \times 9.0 \mathrm { g } ) } { 4 \text { marbles } } = 8.0 \mathrm { g } \text { average }
\nonumber$$
-
The element boron (B) has only two isotopes. Calculate the atomic mass of a large sample of Boron atoms based on the data presented below.
| Isotope | Isotope Mass | Abundance |
| 10B | 10 | 20% |
| 11B | 11 | 80% |
-
Does any single boron atom have the mass you calculated above? Explain.
-
If you grabbed a random atom of boron, what would the mass most likely be?
A scientific instrument called a mass spectrometer can determine the exact mass and abundance of various isotopes. The graph to the right shows some data taken on a large sample of neon (Ne) atoms.
-
Based on the graph, and without looking at a periodic table, estimate the average mass of a large sample of neon atoms. Check the periodic table after making your estimate.
Estimate: _____________
Actual: _____________
Mass Spectrometry
A mass spectrum will usually be presented as a vertical bar graph, in which each bar represents an ion having a specific mass. The x-axis is mass and the y-axis is relative abundance (often represented as percentage where the most abundant is 100).
• Which element is represented in each of these mass spectra?



Summary of Subatomic Particles
-
The Atomic Number on the periodic table tells you the number of _______________.
-
Atomic Mass is found by addition of # _________________ and #_________________.
-
When the # of ____________________ in an atom changes, the charge on the atom changes.
-
Different isotopes have different # of __________________.
-
Why is the atomic mass of elements on the periodic table not a whole number? For example, carbon has a mass of 12.011 instead of 12. Why?
Application Problems:
Mass Spectrometry of Molecules
Modern mass spectrometers easily distinguish (resolve) ions differing by only a single atomic mass unit and thus provide completely accurate values for the molecular mass of a compound.
The mass spectra of two simple compounds: ethylamine, C2H7N (45 amu), and ethanol, C2H6O (46 amu) are shown below.
- Identify which spectrum belongs to which compound. (Hint: Look for peaks on the x-axis that match the molecular weights of the compounds).

Isotopic Ratios and Mass Spectrometry
Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. This is manifested most dramatically for compounds containing bromine and chlorine.
The precise isotopic composition of chlorine and bromine is: Chlorine: 75.77% 35Cl and 24.23% 37Cl
Bromine: 50.50% 79Br and 49.50% 81Br
- The mass spectrum of C6H5Br is shown below. Explain the two peaks at 156 and 158 m/z.
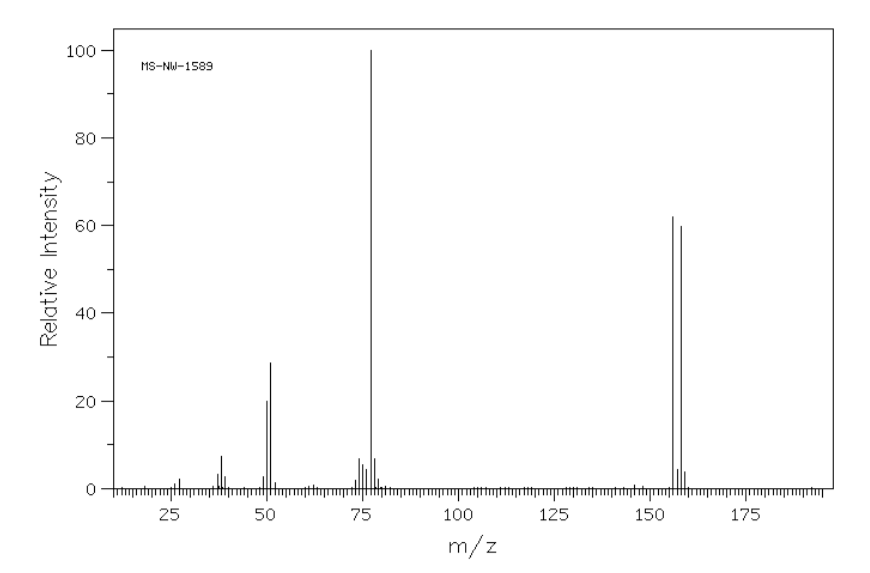
Additional Problems
-
The compound that gave this mass spectrum probably contains:
a) nitrogen b) bromine c) chlorine d) sulfur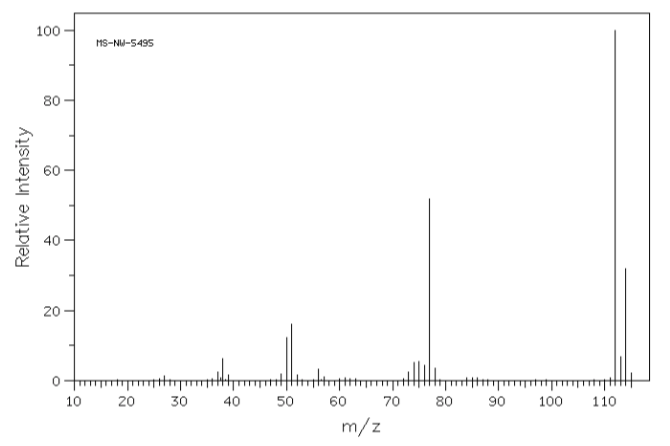
- The compound that gave this mass spectrum probably contains:
a) nitrogen b) bromine c) chlorine d) sulfur

-
How many molecular ions (compounds with different weights) would you expect to see in a molecule with 2 bromines? Explain.
Atomic Orbitals (or where are the electrons?)
Orbitals
-
Whatisanorbital?
-
Howmanyelectronscanbeinoneorbital?
Shown below are drawings of s, p and d orbitals. Because no two electrons on an atom can occupy the same space with the same spin, they are found along different axes.

-
How many nodal planes (changes in phase or shading) are there in each orbital shown above?
-
How does the number of nodes in an orbital relate to the orbital’s energy?
Remember, electrons are waves and in waves, energy increases with higher frequency.
As you go from s to p to d orbitals, the number of nodes increases.
-
Predict the number of nodes that an f orbital would have.
-
Draw an example of an f orbital.
Shells
-
What does the shell number tell you?
-
What is valence shell?
Electron Filling
Here are the 3 electron filling rules:
-
Fill from the bottom up. Start at the lowest energy orbital and go upwards (Aufbau Principle).
-
No more than two! Only two electrons can occupy an orbital, and they must have opposite spin (Pauli Exclusion Principle).
-
Pairuponlyifyoumust.Twoelectronscanbeinthesameorbital,butitcostsa little extra energy. If there is empty orbital orbital at the same energy, better put it there. Align spins in equivalent energy orbitals. (Hund’s Rule)
Pattern of orbital levels/energies
Note
The y-axis on the picture below is energy.

Electronic Configurations & Orbital Filling Diagrams
Main group atoms
- Briefly explain how you can use a periodic table to write electron configurations (numbers of electrons and filling) for atoms.
- Write the electronic configuration for neon.
-
Brieflyexplainthestabilityofthenoblegases.
-
Writetheelectronconfigurationsiliconusingnoblegasconfiguration.
-
-
What is the difference between an electron configuration and an orbital filling diagram?
-
Draw orbital filling diagrams (using arrows and energy levels) for the following elements:
-
O
-
N
-
F
-
Main group ions
An ion is an atom in which the number of electrons does NOT match the number of protons.
Main group atoms: atoms tend to gain or lose electrons to become isoelectronic (same number of electrons) with a noble gas.
- Explain why Na is normally found as Na+. Hint: look at the electronic configurations.
-
Whenanatomlosesanelectrontobecomeacation,whichelectron(from what orbital) would be lost?
-
For anion formation, into which orbital is the new electron placed?
-
Draw the orbital filling diagrams for the following ions: a. C+
-
C+
-
O-2
-
F-
-
Transition metal atoms
4s and 3d levels are almost the same energy levels so the 4s fills before the 3d in most transitional metal atoms.
- Write electron configurations for the following elements. (You can use noble gas configurations to simplify):
-
Ti
-
As
-
Mn
-
-
Predict the electron configuration for Cr.
-
Experimental data has determined that Cr has the electron configuration: 1s22s22p63s23p64s13d5. Fill in the orbital diagram below and use it to explain why chromium fills this way.

-
Knowing what you know about periodic table and the filling for Cr, predict the electron configuration for W.
Transition Metal ions
When transition metals form cations or complexes, the filling changes. orbitals are then lower energy than the 4s orbital.

Thus, valence electrons will typically occupy just the d orbitals in ions/complexes.
For example:
Fe: [Ar] 4s23d6
Fe2+: [Ar] 3d6
Fe3+: [Ar] 3d5
-
Provide the electron configurations for the following transition metal ions:
-
V+
-
Mn+2
-
Zn+
-
-
Organometallic chemists will often refer to a metal in a coordination complex by the number of d electrons. For example, Cu+1 is called d10. What would Cu+2 be called?
Electron Configurations/Orbital Summary
- How many electrons can be in one orbital?
-
What is the valence shell?
-
Label each of the levels below with the correct orbital name

-
List the filling rules in your own words.
-
How can you determine the number of electrons that an ion has?
-
How do you determine the electron configuration of a transition metal atom that has a charge or is in a complex?
Summary: Periodic Table and Electron Configurations
- On the following periodic table, circle and label the s-block, the p-block and the d-block atoms.

-
Label the shells on this periodic table (energy levels).
-
Valence electrons are the electrons in the highest energy level. From the periodic table, how can you quickly determine the number of valence electrons?
-
What column of atoms is the “happiest”? Who does everyone want to be like? Why?
-
Complete the electron configurations for the elements in this partial periodic table.
-
Circle the area of the periodic table that is:
-
s block (last electron filled in an s orbital)
-
p block (last electron filled in an p orbital)
-
d block (last electron filled in an d orbital)
-
-
How do the electron configurations of the elements in a group (column) compare? For example, N, P and As.
Properties of Atoms Periodic Trends: Radii
Atomic radii
The radius of an atom is assumed to be half the distance between the nuclei of two identical atoms when they are bonded together.
- What are the general periodic trends for atomic radii? (Look at the Periodic Table of Radii in the front of this workbook).
-
In a period (across a row):
-
In a group (down a column):
-
-
Using ideas of core charge and Coulomb’s Law, propose a reason for the variation in atomic radii:
-
In a period (across a row):
-
In a group (down a column):
-
-
Which atom would you expect to be larger?
Ca or Ga? Si or Sn?
Ionic radii
-
The radius of an atom changes as the charge on the atom changes.
Atom/Ion Na Na+ Mg Mg2+ O O2- F F- Atomic Radius (pm) 186 102 160 72 73 126 72 120 -
If an electron is added to an atom, the charge becomes [ positive / negative ]. This [ cation / anion ] is now [ smaller / larger ]

-
If an electron is removed from an atom, the charge becomes [ positive / negative ]. This [ cation / anion ] is now [ smaller / larger ].

-
Propose a reason for this radius change (Hint: Think about Coulomb).
-
Which of these represents a Rb+ atom? a Br- atom?

-
Arrange the following isoelectric ions (same number of electrons) in order of decreasing size:
Na+, F–, Al3+, Mg2+, O2–
Periodic Trends: Ionization Energy and Electronegativity
Ionization Energy
Ionization energy (Ei) is the amount of energy required to remove the highest- energy electron from a neutral atom in the gaseous state.

Note
All the values are positive, meaning that energy is always required to remove an electron from an atom.
-
Which atoms are least likely to give up an electron?
-
Which atoms are most likely to give up an electron?
-
Explain your observations about which atoms are likely/unlikely to lose an electron.
Multiple Ionizations
Generally the (n+1)th ionization energy is larger than the nth ionization energy.
-
In other words, it requires (more or less) energy than to remove each successive electron.
-
Explain a reason for this trend.
While the successive ionization energies increase in increments. Occasionally, large jumps in the successive molar ionization energies occur.
- Looking at the table of successive ionization energies, circle the largest ionization energy for each atom.

-
Write the electron configuration for the ion that is least willing to give up its electron in each case.
-
For example, it is difficult to convert Na1+ -> Na2+ . What is the electron configuration for Na1+ ?
-
Mg+2:
-
Al+3:
-
Si+4:
-
-
Propose a reason for why this ionization energy is so much larger.
Minor Irregularities in Ionization Energies
There are minor irregularities across a row in the periodic table (group 2 and group 6).
Group 2:
- Draw orbital filling diagrams for the following atoms:
Be Be+
B B+
The Ei for beryllium (Be) is larger than that of boron (B).
- Why would it be easier to remove an electron from B than Be?
Group 6:
- Draw orbital filling diagrams:
N N+
O O+
Similarly, the Ei for nitrogen (N) is larger than that of oxygen (O)
- Why would it be easier to remove an electron from O than N?
Electronegativity
Electronegativity (EN) is the ability of an atom in a molecule to attract electrons or electron density towards itself.
An atom's electronegativity is affected by
-
Theatomicnumber
-
Thedistanceofvalenceelectronsfromthechargednucleus.

-
What is the trend in EN as you proceed from left to right in a period?
-
Provide a reason for this trend.
-
What is the trend in EN as you proceed from top to bottom in a group?
-
Provide a reason for this trend.
-
How do the periodic trends for ionization energies compare to the trend for electronegativity? Why are these trends related?
Summarize Periodic Trends
- On the outline of the periodic table, put arrows for trends: Include the following trends:
Atomic radii
EN
Ionization Energy
-
Explain each trend.
Application Problems
-
Which of the following represents a Ca atom? a Sr atom? a Ba atom?
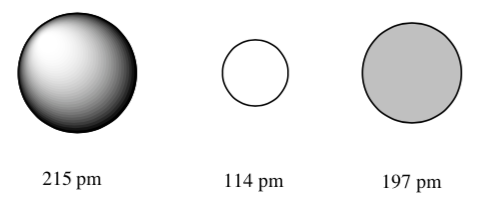
-
Arrange the following isoelectric ions (same number of electrons) in order of decreasing size:
Cl–, Ca2+, S2–, K+, Sc3+
-
Voltage-gated sodium channels are part of the cell signaling system. A nearby cell membrane potential (a charge separation across the membrane) induces a change in the shape of a protein surrounding the channel. The channel opens and a sodium cation is allowed into the cell, but not its counterion. This charge separation induces another channel to open, and so on, so that the charge is propagated along the cell membrane. In this way, electrical signals can be transmitted along a neuron.
-
Why does the sodium channel let the sodium ion into the cell, but not a chloride ion?
-
Why does the sodium channel let the sodium ion into the cell, but not a postassium ion?
-
-
Atomic Absorption
Atomic absorption spectroscopy is a technique used to determine the presence and concentration of a specific metal element in a sample.
In AA, the electrons of the atoms from the sample metal can be promoted to a higher orbital for a short amount of time by absorbing a set quantity of energy (i.e. light of a given wavelength). Atoms generally have several possible transitions.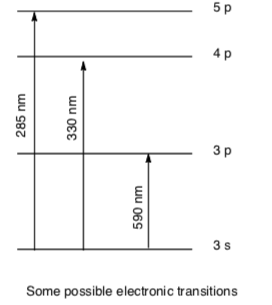
Using this figure:
-
Which transition takes the most energy?
3s -> 3p 3s -> 4p 3s -> 5p
Transitions result from exciting an electron from a filled (or partially filled) orbital to an empty or partially filled orbital.
-
Which atom(s) could have an absorption at 590 nm:
Li Cd Mg N Si Ar
-
Which atom(s) could have an absorption at 330:
Li Cd Mg N Si Ar
-

