16: Network Solids
- Page ID
- 142848
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Network Solids Structure and Properties
Packing of Network Solids
Network solids are made of covalent bonds in a continuous network. In a network solid, there are no individual molecules and the entire crystal may be considered a macromolecule.
-
Predict melting points for network vs. molecular solids.
Graphite
Graphite is a molecular form of carbon (shown below). In its appearance and feel, graphite is similar to molybdenum disulfide.
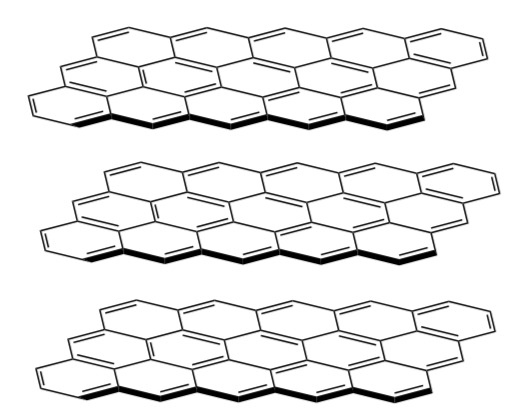
-
What is the molecular geometry of each carbon?
-
What intermolecular force(s) is holding the layers of this structure together?
-
Graphite is often used in pencils as the “lead”. Using this picture of its’ molecular structure, explain why a pencil can leave layers of graphite as marks on paper.
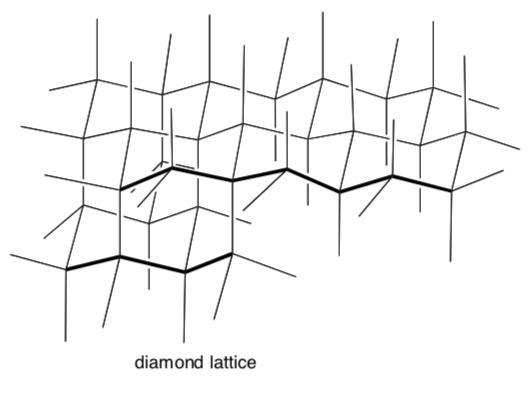
Diamond Lattice in Covalent Network Solids
Many network solids with tetrahedral centers adopt the “diamond lattice structure”.
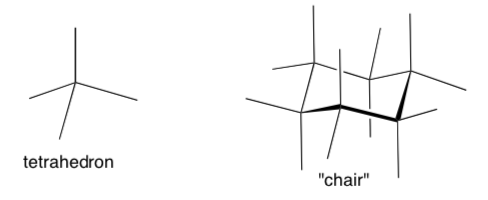
- Trace a tetrahedron on this "chair" above.
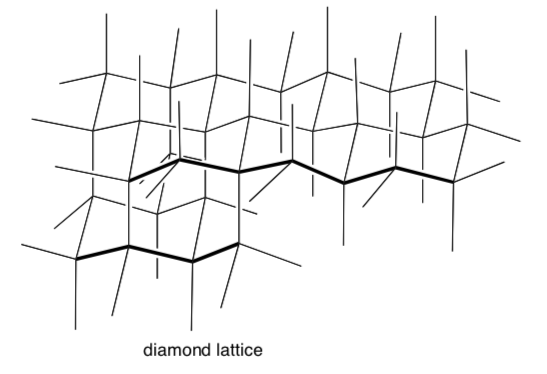
For diamond, each tetrahedral center is a carbon atom.
-
Trace a tetrahedron on this diamond lattice.
-
Trace a chair on this diamond lattice.
Water (ice) adopts a structure based on a diamond lattice.
- Trace out a chunk of ice using the diamond lattice:
-
Each tetrahedral center is an oxygen atom
-
Fill in several oxygen atoms
-
Fill in several covalent H-O bonds
-
Fill in several hydrogen bonds
-
-
Why does ice float on cold water?
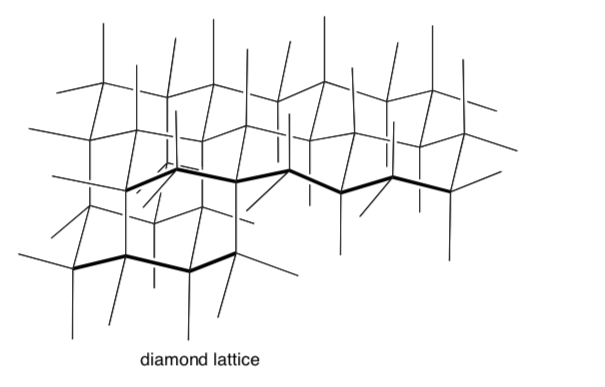
Most sand and rock is composed chiefly of silica. The empirical formula for silica (SiO2) adopts a structure based on a diamond lattice
- Trace out a chunk of silica using the diamond lattice (shown below):
-
Each tetrahedral center is a silicon atom.
-
Fill in several silicon atoms.
-
Fill in several oxygen atoms between silicon atoms.
-
-
What happens at the edge?
-
What atoms are found on the edge of the silica?
-
How many bonds do they typically have?
-
Fill any empty valences with hydrogens.
-
-
What is the polarity on the inside of a chunk of silica? Non-polar or Polar
-
What is the polarity on the surface of a chunk of silica? Non-polar or Polar
-
What do the geometries of oxygen, carbon and silicon have in common? Why are these solid structures so similar?
Zeolites
Zeolites are microporous, aluminosilicate (made from alumina, silica and oxygen) network solids. There are a variety of zeolites with different structures.
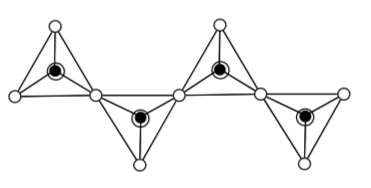
The building block for zeolites is a tetrahedral silicon or aluminum with four oxygens covalently bound:
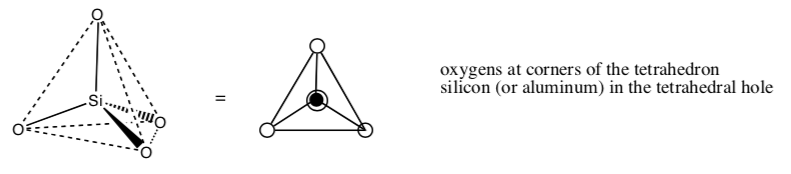
-
What is the formal charge on the silicon when bonded to four oxygens?
-
What is the formal charge on the aluminum when bonded to four oxygens?
When this network is continued:
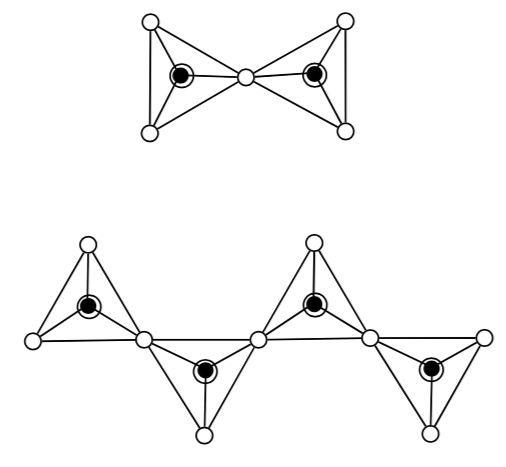
These building blocks can be assembled in different patterns. The hexagon is common.
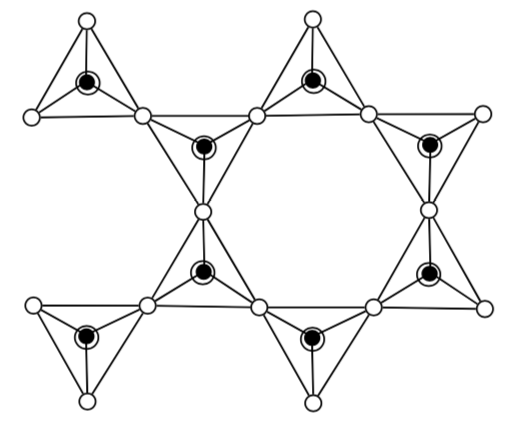
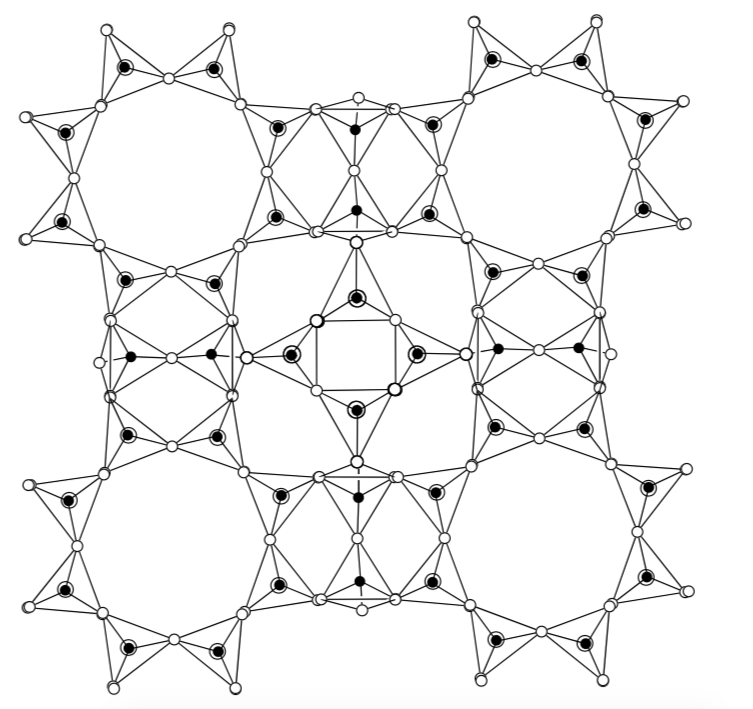
To simplify, the structures are also drawn with a skeleton format. In this structure, each corner and each end of the stick is a tetrahedron of silicon (or aluminum) bonded to four oxygens.
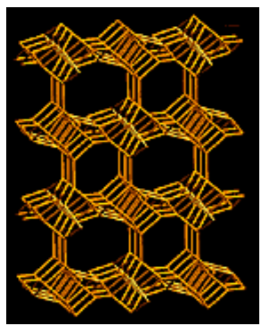
In 3D, you can begin to imagine long channels forming.
The channels in the zeolite structures can bind cations, such as Na+, Ca2+, Mg2+ and others. These positive ions are rather loosely held and can readily be exchanged for others in a contact solution.
-
Why do zeolites bind cations?
-
What type of IMF holds the cations in place?
Zeolite Applications
-
Ion Exchange in Water Softeners
An example of a soap molecule is sodium stearate (pictured below).
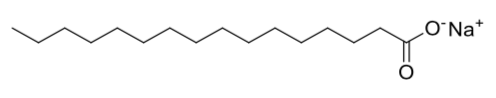
Most conventional water-softening devices depend on a process known as ion- exchange in which "hard" ions like Ca+2 trade places with Na+1 or K+1 ions that are loosely bound to a zeolite.
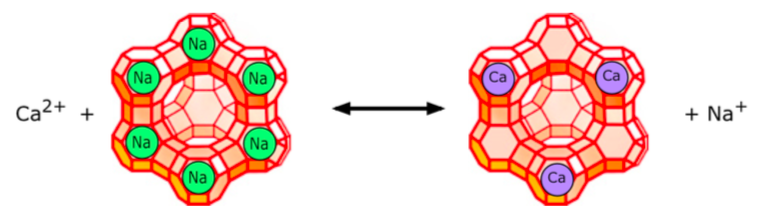
The illustration depicts a negatively-charged zeolite to which [positive] sodium ions are attached. Calcium or magnesium ions in the water displace sodium ions, which are released into the water.
As the zeolites become converted to their Ca2+ form, they gradually lose their effectiveness and must be regenerated.
-
In “hard water”, there are Ca+2 ions. If the calcium ions replace the sodium ions, what will the soap look like? Remember, we need to balance the charges.
-
Calcium salts of most soaps are not soluble in water and these salts precipitate out of solution as “scum” or “bathtub ring”. How will this affect the effectiveness of your soap?
- Why does the Ca+2 replace the Na+?
-
Why are there only 3 Ca+2 vs 6 Na+ bound in the zeolite shown above.
-
Suggest a method for regeneration of the ion exchange cartridge in your water softener.
-
-
Another use for zeolites is to remove toxic heavy metal cations from wastewater. The natural zeolite, clinoptilolite, will the selectivity bind heavy cations in the following order:

-
Rank these four cations by size (Look at atomic radii).
-
This zeolite was not effective at removing Hg2+. Suggest a reason.
-
-
Sometimes zeolites are used to separate molecules because only molecules of certain sizes and shapes can pass through.
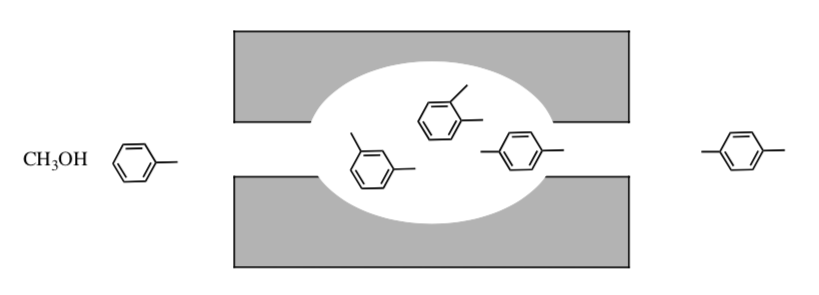
In the acid-catalysed alkylation of methylbenzene, all three dimethylbenzenes are produced in the zeolite channel (cartoon shown below). Only one can diffuse out. This is called product selective catalysis.
-
Explain why only the 1,4 dimethylbenzene is released.
-
-
Molybdenum Sulfide Grease
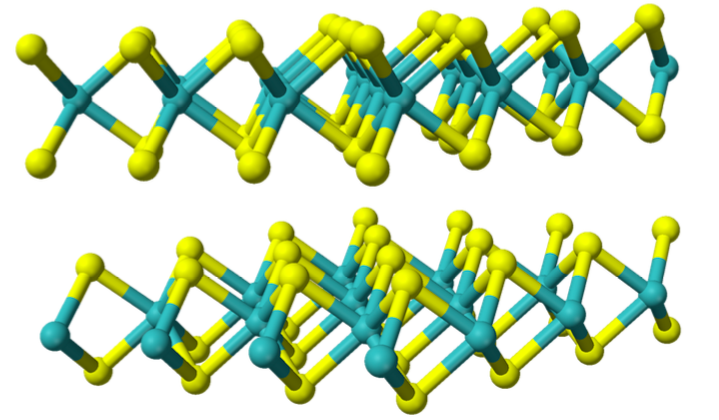
Molybdenum disulfide, MoS2, is a black crystalline network solid that occurs as the mineral molybdenite.
-
What is the Coordination Number of each molybdenum (darker atoms)?
-
What is the Coordination Number of each sulfur (lighter atoms)?
-
What intermolecular force(s) is holding the layers together?
-
This compound is widely used as a lubricant because of its low friction properties, sometimes to relatively high temperatures. Using this picture of its’ molecular structure, explain why this network solid makes a good lubricant.
-
-
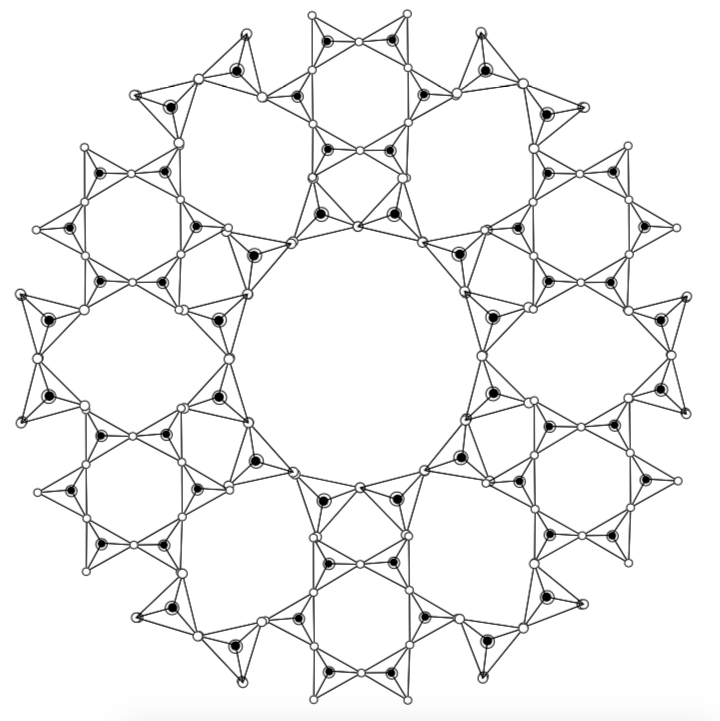
Redraw the Zeolite structures shown below in skeleton/line drawings:
-
Application Problem
Marcus S. Niepel, Bodo Fuhrmann, Hartmut S. Leipner, and Thomas Groth, Nanoscaled surface patterns influence adhesion and growth of human dermal fibroblasts, Langmuir,2013, 29 (43), 13278–13290.
Most sand and rock is composed chiefly of silica. The empirical formula for silica is SiO2 and it adopts a structure based on a diamond lattice. The surface on the crystalline solid can be functionalized and is used in a variety of applications.
-
Trace out a chunk of silica using the diamond lattice (shown below):
-
Fill in several silicon atoms.
-
Fill in several oxygen atoms between the silicon atoms.
-
What is the geometry around the silicon atom?
-
Is the SiO2 surface polar or nonpolar?
-
-
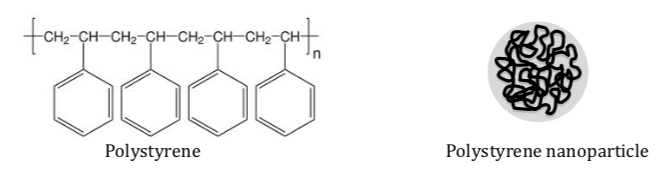
Polystyrene consists of repeating units of styrene and can be spun to create nanoparticles.
-
Is polystyrene polar or nonpolar?
-
What are the intermolecular forces holding the nanoparticles together?
Ion-ion ion-dipole dipole-dipole Hydrogen binding London dispersion
-
Sketch a monolayer of hexagonally closest packed polystyrene nanoparticles as viewed from above.
-
What are the strongest intermolecular forces between polystyrene and a silica surface, shown below?
ion-ion ion-dipole hydrogen binding ion-induced dipole dipole-dipole London dispersion dipole-induced dipole

-
-
Nanoscale lithography (NSL) is used to create a nanopatterned silicon surface, in which nanoparticles are placed on a flat silicon dioxide surface and used as a mask for subsequent deposition of gold. The remaining gold and silicon surface can then be functionalized with different molecules.

The remaining gold structures are deposited between the nanospheres.
-
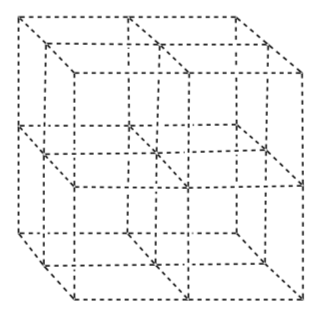
Draw the polystyrene nanoparticles as a face-centered cubic lattice.
-
What holes do the gold atoms occupy?
-
-
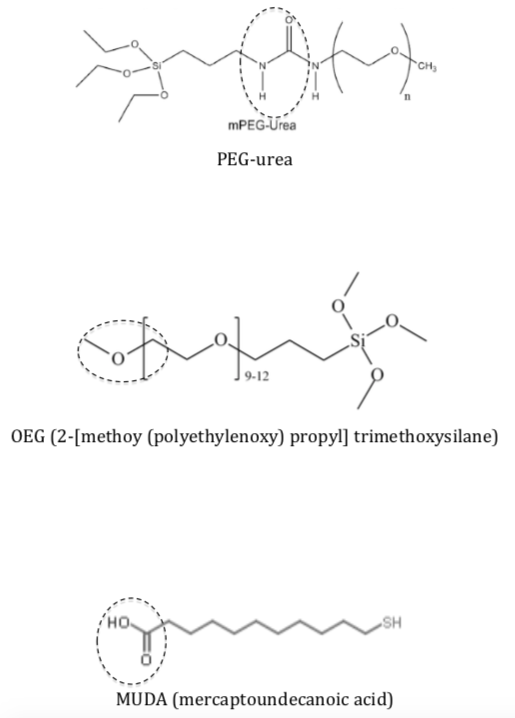
Self-assembled monolayers (SAM) can be used to modify the silica surface and the surface of the gold nanostructures to obtain chemistry of interest; this is used to control the interactions of the surfaces with biological molecules. Below are three SAM used in the study.
-
Label the circled functional groups.
-
-
Fibronectin is an extracellular protein that provides a matrix for human cells to adhere to. Scientists often try to adhere fibronectin to a non-biological surface so that cells can then stick to the fibronectin. Cartoon images of fibronectin and two examples of modified silica surfaces are shown below.
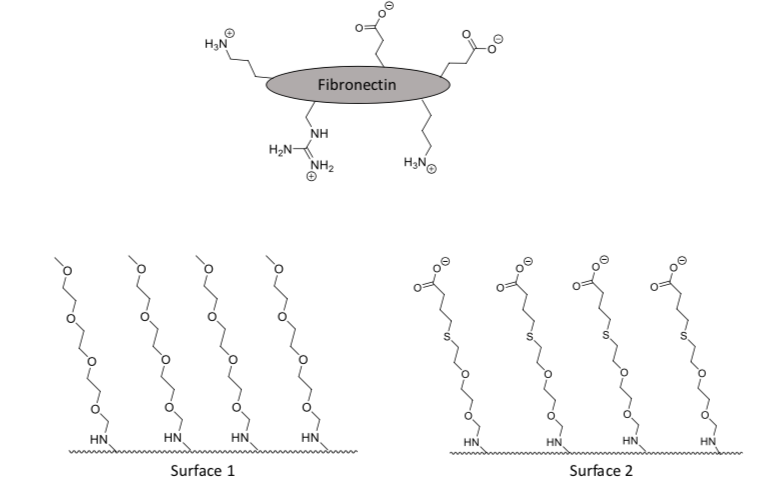
-
What is the strongest type of intermolecular force that fibronectin would experience with Surface 1?
Ion-ion ion-dipole dipole-dipole hydrogen bond ion-induced dipole dipole-induced dipole induced dipole-induced dipole
-
What is the strongest type of intermolecular force that fibronectin would experience with Surface 2?
Ion-ion ion-dipole dipole-dipole hydrogen bond ion-induced dipole dipole-induced dipole induced dipole-induced dipole
-
Which surface would fibronectin preferentially bind to? Explain your answer.
-
-
Human cells can connect to fibronectin via the cell membrane protein known as integrin. Integrin has arginine and aspartic acid residues where it binds to fibronectin. A cartoon image of this association is shown below.
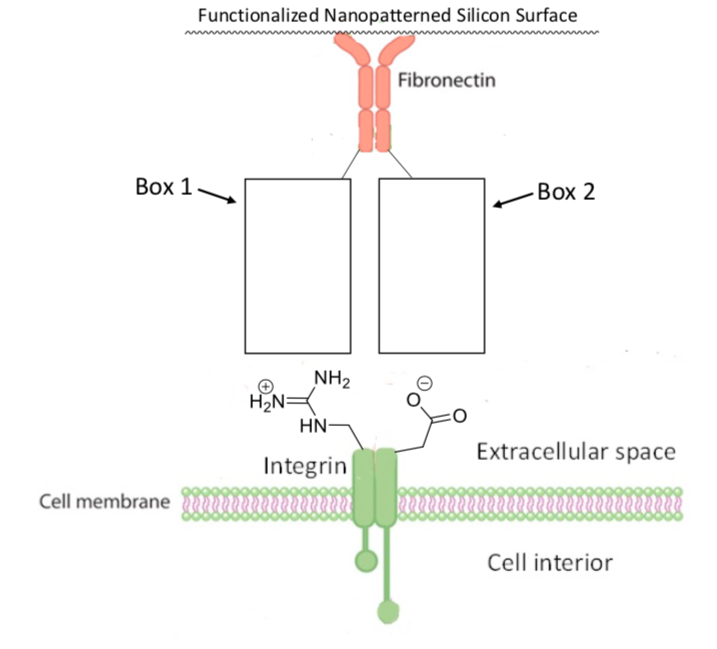
-
In Box 1, draw in an amino acid residue that would bind strongly with the arginine residue of integrin.
-
In Box 2, draw in an amino acid residue that would bind strongly with the aspartic acid residue of integrin.
-
Based on the amino acid residues that you drew in the boxes above, what is the strongest type of intermolecular force that would hold fibronectin and integrin together?
Ion-ion ion-dipole dipole-dipole hydrogen bond ion-induced dipole dipole-induced dipole induced dipole-induced dipole
-
-
An unmodified silica surface produces the largest thickness increase of protein while long chain mPEG-Urea efficiently prevented absorption.
-
Explain using intermolecular forces.
-
How would the chain length of PEG affect protein attraction?
-
-
What is the purpose of controlling surface adhesion, i.e. why would you want adhesion, growth or proliferation of cells only in certain areas?
Silica Gel Chromatography Application Problem
Jiang, Zhang, Liu, Dong and Liu, Journal of Natural Products, 2009, 72, 1405-1409.
-
The basidiomycete Hexagonia speciosa is a fungus that is found in the tropical zones of China such as Yunnan and Hainana Provinces. The secondary metabolites were isolated from this fungus with the hope of finding compounds with biological activities such as antifungal, antibiotic or anti-tumor.
-
Based on your understanding of the structure of silica gel, SiO2 (previous page), the column packing material is:
Polar Non-polar
-
Draw the Lewis dot structure of chloroform:
CHCl3
-
Relative to the silica gel, chloroform (solvent) is:
Polar Non-polar
-
Which type of compound will elute first from a Silica Column with chloroform as eluent?
Polar Non-polar
-
-
Twelve compounds were purified from H. speciosa using column chromatography. Shown below are six of the compounds.
-
Number the compounds in order in which they would elute from a Silica Column with chloroform as the eluent.
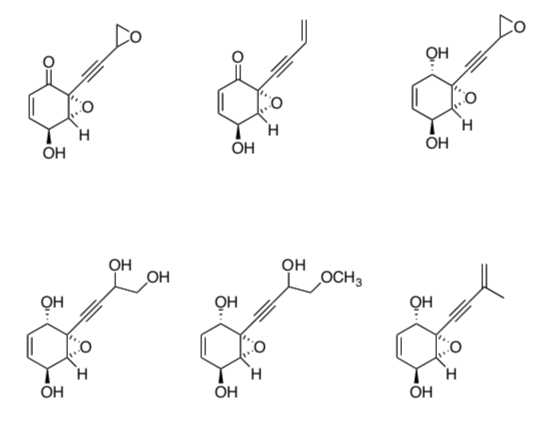
-