17: Coordination Compounds
- Page ID
- 142877
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Coordination Compounds: Structures of Transition Metals
In a coordination compound, a central metal ion is attached to a group of surrounding molecules or ions (called ligands).
Coordination Numbers and Geometry
Coordination number gives rise to characteristic shapes, just as the number of electron domains helped us predict shape in main group molecular compounds.
-
Complete the following table:
CN Geometry 2 3 4 5 6
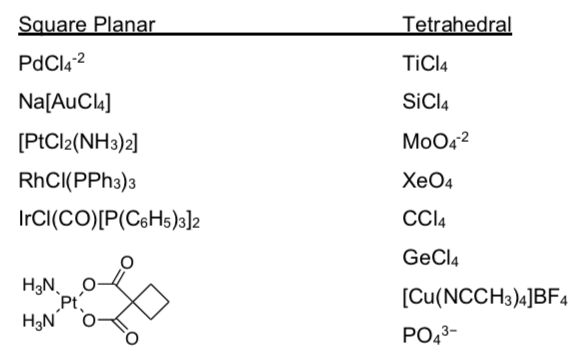
There are two possible shapes for coordination number 4: tetrahedral and square planar. Tetrahedral is often favored.
-
Explain why tetrahedral would be favored using steric considerations. Looking at the data below, can you find a trend for compounds that tend to be square planar instead? Consider where the metal is on the periodic table.
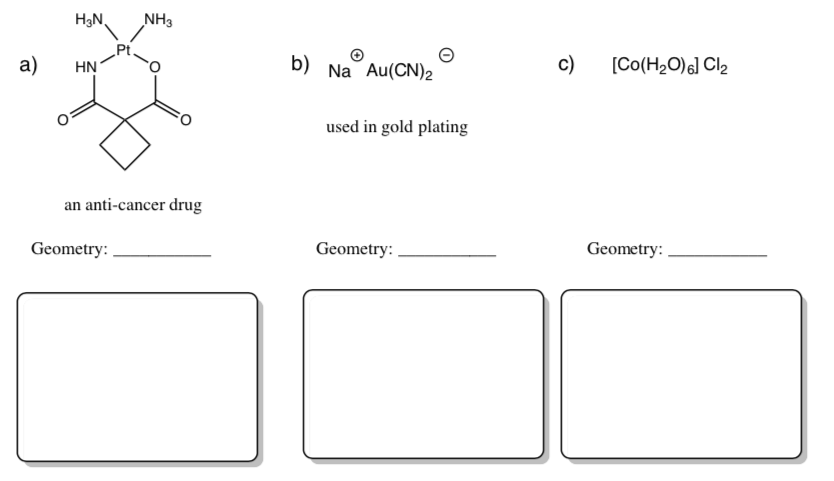
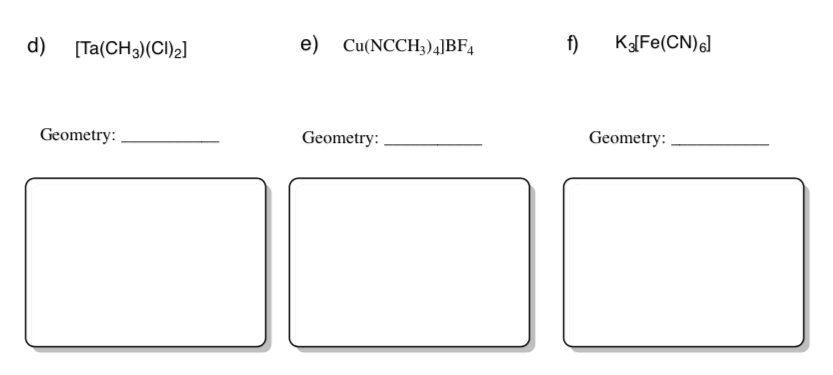
Practice Predicting Geometry and Drawing Coordination Complexes
-
The coordination complex is inside the brackets.
-
A ligand that binds to the metal is inside the parentheses.
-
In front of the brackets is a cation. Behind the brackets are negative anions. Cations and anions float nearby but do not affect the coordination number.
- Predict the geometry of each compound.
-
Draw the compounds in the predicted geometry using wedges and dashes.
Ligand Binding
A dative bond (also called a coordinate covalent bond) is a bond where one species provides both electrons for the covalent bond.
-
A ligand donates at least one pair of electrons to the metal.
-
Ligands may be anions or neutral.
-
Draw structures of these neutral ligands and circle the lone pair that will be donated:
H2O (aqua) NH3 (ammine) CO (carbonyl)
-
Draw structures of these anionic ligands and circle the lone pair that will be donated:
Cl- (chloride) CH3- (methyl) OH- (hydroxide) H- (hydride)
Polydentate Ligands
Denticity is the number of donor atoms per ligand. If there is only one donor atom the ligand is monodentate (meaning “one – toothed”).
Polydentate ligands are also known as chelating agents. These agents bond to metal ions and “trap” them as very stable complexes.
-
What would bidentate imply?
-
What would tridentate imply?
-
What would tetradentate imply?
-
What would polydentate imply?
-
Ethylenediamine (H2NCH2CH2NH2 , often abbreviated as en) is a common ligand. What are the donor atoms on this ligand? How many times will this ligand bind?
-
Draw Ni(en)(H2O)4+2
-
Draw Mg+2 complexed with propylenediamine (H2NCH2CH2CH2NH2). What shape will this complex adopt?
-
Hydrazine (NH2NH2) has two lone pairs but is NOT bidentate. Explain why.
-
Determine how many times each of these ligands might bind a metal:
-
Label the donor atoms:
-
Coordination Compounds in Biology
Donating one or more lone pairs to a metal ion is common in biology.
- Show the complexes that result in the following cases and describe the geometry of the metal in the new complex.
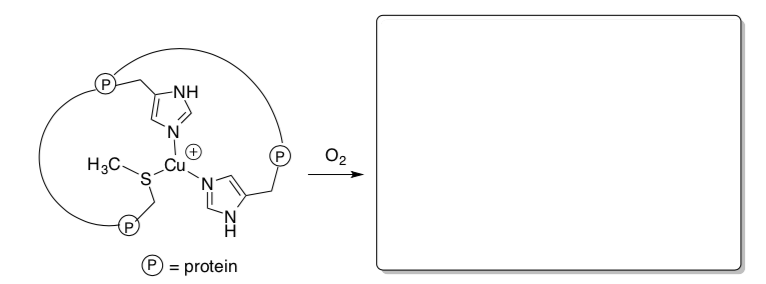
Dopamine beta monooxygenase, a protein that binds an oxygen molecule and uses it to modify other proteins so they can become important signaling molecules
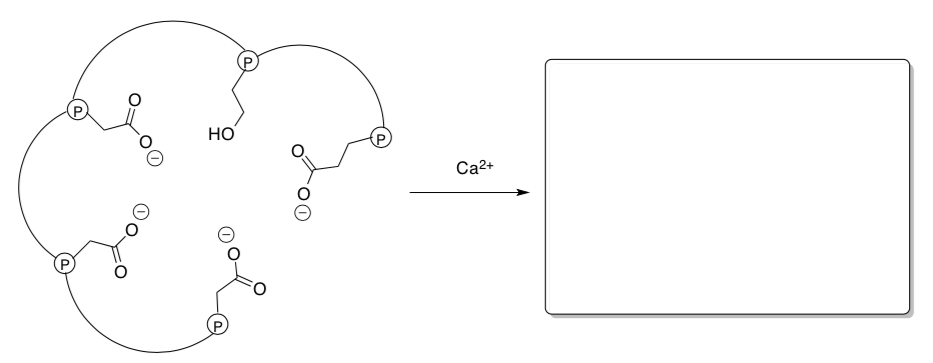
Calmodulin, a little worker protein that binds to other proteins to get them going. It needs calcium to get started. When it binds calcium, a "conformational change" results because the molecule has to twist to reach that calcium. In its new shape, it can fit together with other proteins.
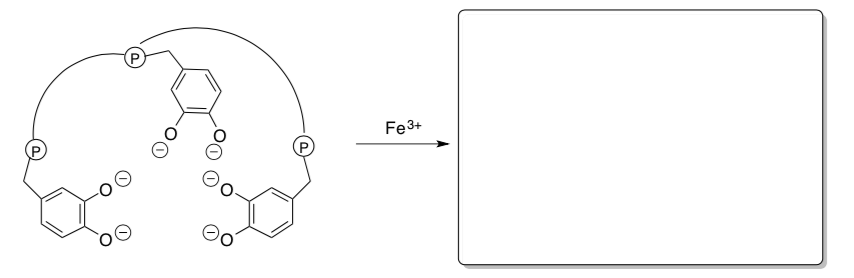
Enterobactin, a protein secreted by certain bacteria to gather iron from their environment.
Electron Counting on Coordination Complexes
Electron count on a metal in a complex can be accomplished as follows:
-
Decide whether any ionic ligands are present (just cover up the metal, assume the donor atom in the ligand has an octet, and determine whether that atom has a formal charge). If so, adjust the charge on the metal atom to keep the overall charge balanced.
-
Count the number of electrons on the metal, given its oxidation state (charge on the metal).
-
Count the number of electrons donated by ligands.
-
Total the electrons.
Let’s try an example: (PPh3)4Pd
- Draw this compound.
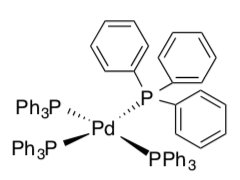
- Disconnect the ligands assuming the pair in the dative bond remains with the ligand.
-
Calculate the charge on each ligand.
-
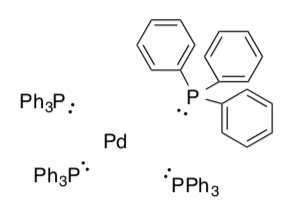
-
Determine the charge on the metal.
-
Fill in the following table:
Charge on the ligands :__________
Valence electrons on Metal:___10____
Charge on the Metal :____0____
Revised Count on the Metal (accounting for charge): ____10____
Number of electrons donated from the ligands :___________
Two per dative bond to the metal
Total electrons in this complex :__________
Let’s try a more complicated example: K[Ru(CN)4(NH3)2]
-
Draw this compound (remember the complex is inside the brackets).
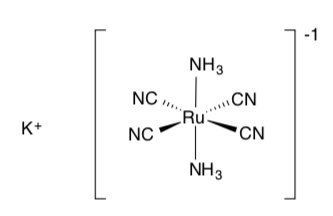
-
Disconnect the ligands assuming the pair in the dative bond remains with the ligand.
-
Calculate the charge on each ligand.
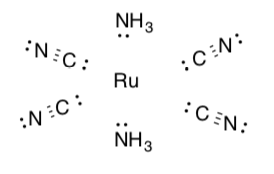
NH3 ____________
CN _________
Total charge on ligands: _____-4_____
-
-
Determine the charge on the metal.
Charge on complex = charge on metal + charge on ligands
-1 = ________ + (-4)
-
Fill in the following table:
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Two per dative bond to the metal
Total electrons in this complex :__________
-
Count the electrons on the metals in these complexes.
[Mo(CO)6]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Ni(CO)4]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Co(NH3)6] (Cl)3
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[(PPh3)2Rh(CO)Cl]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
K3[Fe(C2O4)3] (hint: bidentate oxalate ligands)
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Ni(en)2(Cl)2]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Re(CO)5(PF3)]Cl
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[(PPh3)3RhCl] (Wilkinson's catalyst)
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[(NH3)2PtCl2] (cis-platin)
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Pd(en)2(NO2)2](PF6)2
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
[Co(NH3)3Cl3]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
18 Electron Rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes.
-
How many valence shell orbitals do transition metals have?
-
Why would transition metals want 18 electrons?
Although the majority of metal complexes do not satisfy the 18-electron rule, the rule Predicts formulas for many organometallic complexes of the Cr, Mn, Fe, and Co triads.
- Compounds that obey the 18 VE rule are typically "exchange inert." Complexes with fewer than 18 valence electrons tend to show enhanced reactivity. Explain.
Complexes with bulky ligands often do not complete the 18 e- configuration.
-
Draw these complexes in the correct geometry and count the electrons.
Co(norbornyl)4 Pt(PtBu3)2 ((CH3)3CCH2)3TaCl2
Note:
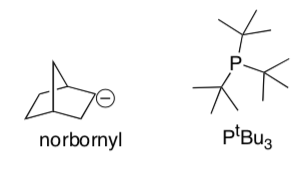
- What prevents these compounds from binding to more ligands and achieving a full valence shell?
Isomers of Coordination Complexes
Linkage isomers occur when there is a choice between connecting the metal to one atom or another atom in the same ligand.
-
Draw the two best Lewis structures for SCN- (same connectivity) and assign formal charges to each atom.
-
In SCN- , which atoms are most likely to donate a pair of electrons. Explain your reasoning.
-
Show two pictures of SCN- binding to a metal (M).
Geometric Isomers in Coordination Complexes
We have looked at cis and trans before in the context of carbon compounds.
-
Define cis and trans:
This same type of relationship can also be applied to transition metal complexes.
-
Draw the two geometric isomers of the square planar complex Pt(NH3)2Cl2
-
Which structure would be considered cis and which one trans? Why did you label them as such?
-
Draw the two geometric isomers of the octahedral complex [Co(NH3)4Cl2].
-
Which structure would be considered cis and which one trans? Why did you label them as such?
Another diastereoisomeric relationship is fac and mer. These occur when there are three identical ligands
-
Draw the geometric isomers of the octahedral complex [Co(NH3)3Cl3].
-
How would you describe the relationship between the two (i.e. what is different between the two)?
Stereoisomers
- Review: Define enantiomer.
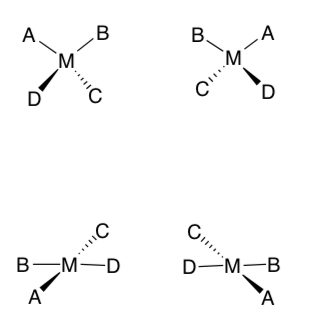
Square Planar vs Tetrahedral
Compare the two mirror images of a metal complex in four coordinate shapes -- tetrahedral and square planar.
-
Label the tetrahedral pair and the square planar pair.
-
Are these pairs of enantiomers? Or pairs of identical complexes?
-
If achiral, show a plane of symmetry.
Octahedral Stereoisomers
Chiral octahedral complexes, with chelating ligands can have two different configurations (as shown above). Δ (delta) is used to indicate clockwise rotation of the helix. While Λ (lambda) is used to indicate anticlockwise rotation of the helix.
- Label each of the two complexes below with Δ (delta) or Λ (lambda).
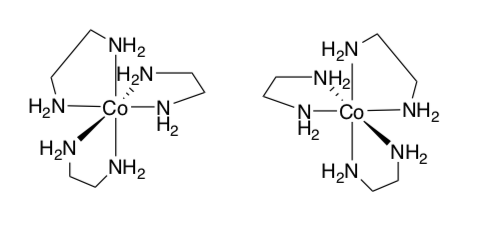
Octahedral Stereoisomers
-
Will any of these pairs of octahedral complexes be chiral?
-
Draw a plane of symmetry through any complex that is achiral.
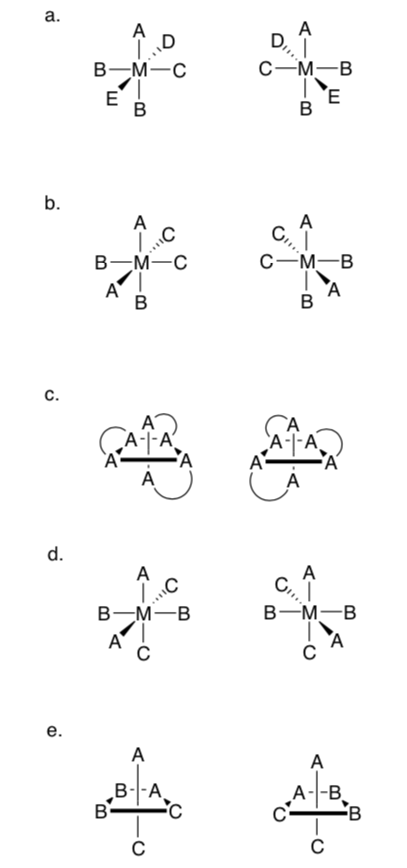
Finding all the isomers in Octahedral Transition Metal Complexes
The “trans pair” system method is used to determine the number of possible isomers (geometric and optical) for an octahedral transition metal complex.
Trans Pair System:
-
Draw 1 isomer with the ligands arranged in any order.
-
Remember bidentate ligands can only occupy cis sites.
-
-
Write out the 3 pairs of trans ligands.
-
Switch a pair of cis ligands and again write out the 3 pairs of trans ligands.
-
If the 2 isomers do not have the same pairs of trans ligands then they are geometric isomers of one another.
-
-
Examine each geometric isomer for an internal plane of symmetry (or draw mirror image).
-
If there is an internal plane, or its mirror image is superimposable, then it does not have an enantiomer.
-
If there is no internal plane of symmetry, or its mirror image is non-superimposable, then it does have an enantiomer.
-
-
Use the trans pair system to determine the number of geometric isomers for [Cr(H2O)3(OH)2Cl].
-
Which of the isomers of [Cr(H2O)3(OH)2Cl] have enantiomers?
Additional Practice with electron counting
-
For each of the following complexes, draw all geometric isomers and label them using the “trans pair” naming system.
-
Indicate which exist as a pair of enantiomers.
-
Determine the charge on each metal and its valence electron configuration.
-
Determine the total electron count on each complex (metal valence electrons + electrons donated from ligands).
-
[Mn(NH3)2Cl2F2]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
-
[Ni(en)(ox)(H2O)(NH3)]
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
-
[Co(NH3)2(en)2]3+
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
-
[Pt(en)2ClBr]2+
Valence electrons on Metal :__________
Charge on the ligands :__________
Charge on the Metal :__________
Revised Count on the Metal (accounting for charge) :__________
Number of electrons donated from the ligands :__________
Total electrons in this complex :__________
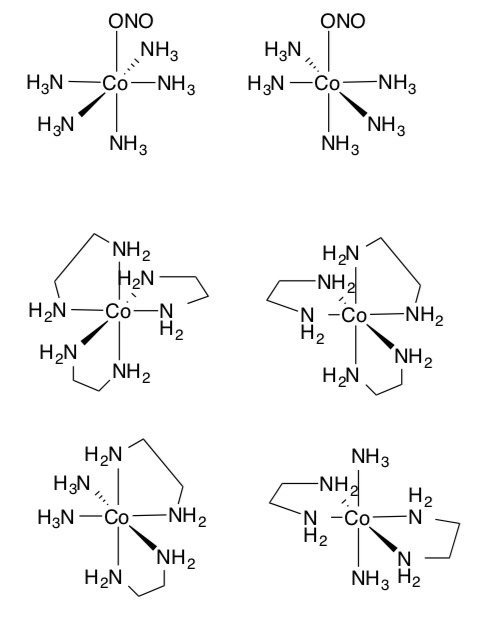
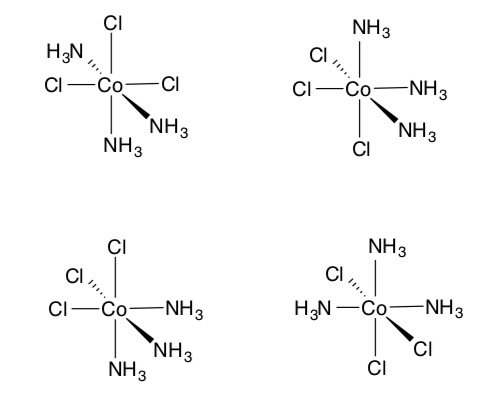
Additional Practice with Types of Isomers
- Label the type of isomeric relationship between these compounds (fac/mer, cis/trans, enantiomers, linkage or identical)
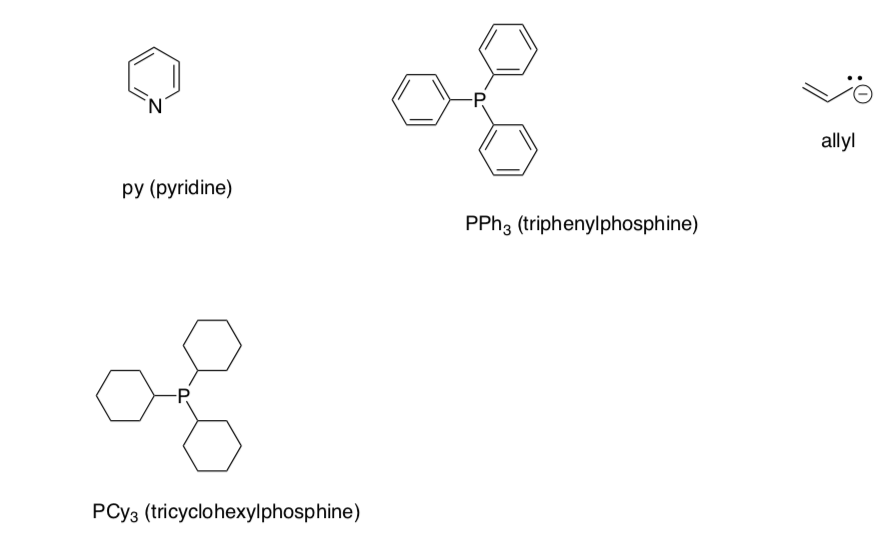
Ligand Reference Table 1
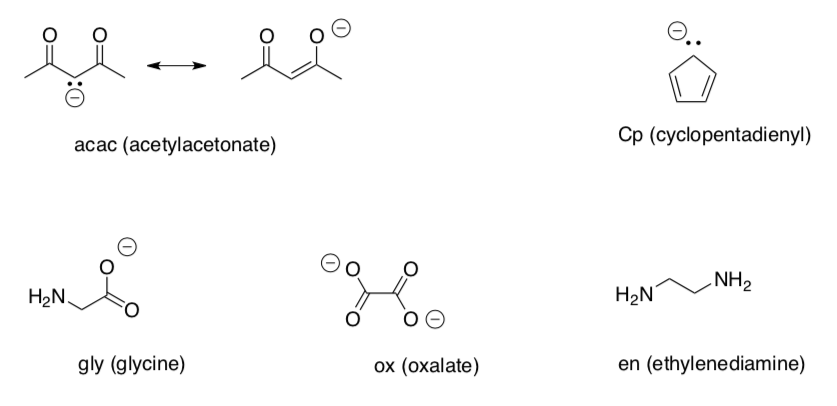
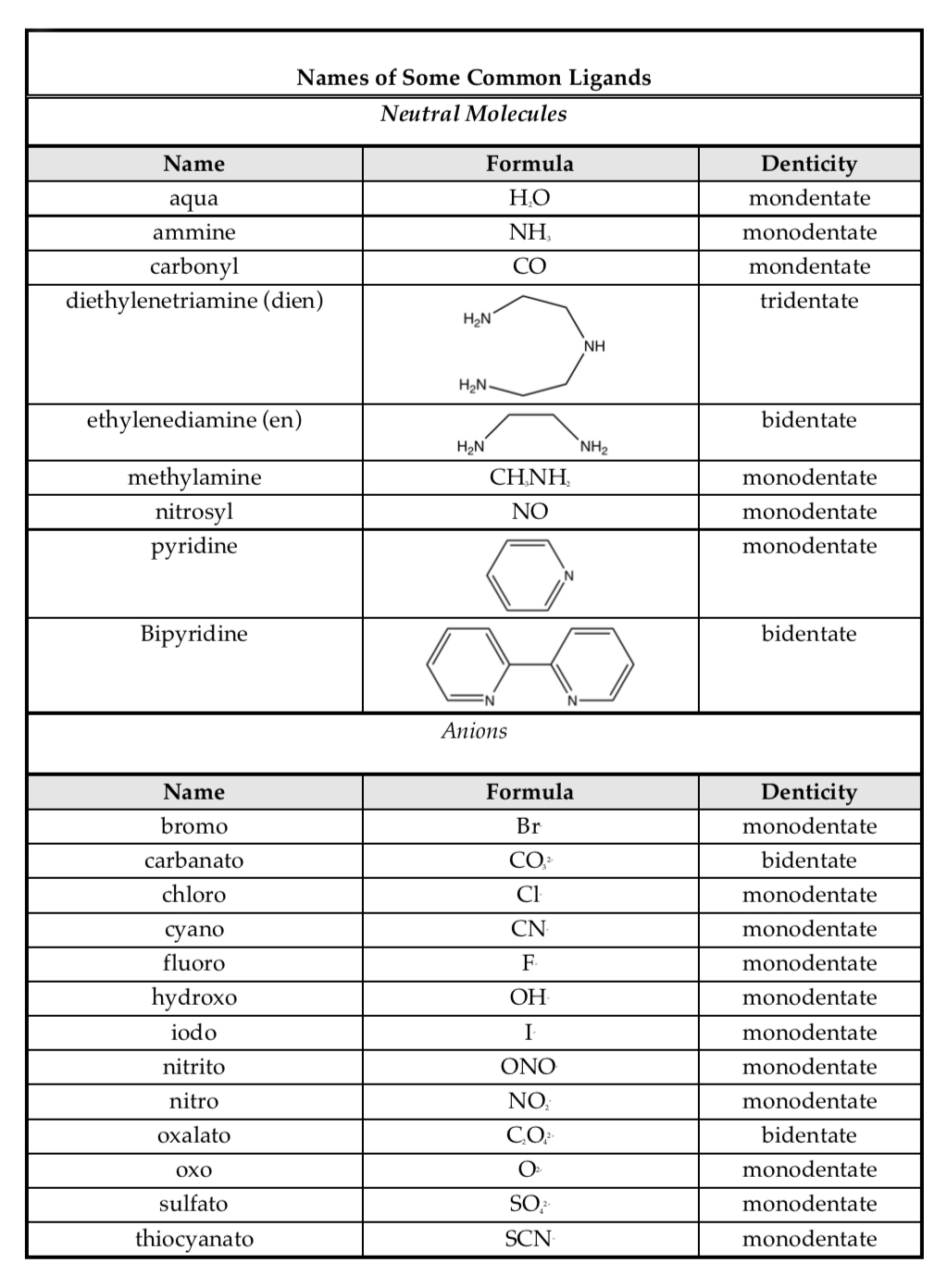
Ligand Reference Table 2
Summary
Chemistry is based on the 3D-shapes of molecules. How one structure is related to another is often very important in predicting the differences in reactivity.
-
Develop a concept map that shows the relationships (similarities and differences) for all of the isomers (main group or coordination complexes) that you have learned about.
Constitutional Linkage
Conformational Fac/mer
Enantiomers R/S
Diastereomers Geometric isomers
Meso Cis/trans
-
How do you determine if a ligand on a coordination complex is charged?
-
List the steps for counting electrons In a coordination complex.
-
What is coordination number? Coordination geometry?
Application Problems
-
One way to treat heavy metal poisoning (such as excess Pb2+, Hg2+ or Cd2+ in the blood) is to use EDTA4- (also known as ethylenediaminetetraacetate ion. The metal complex can then be harmlessly excreted by the kidneys. This ligands is also used to treat pet birds since they often ingest lead or zinc from the solder on their cages.
-
Draw EDTA (shown on an earlier page) bound to one of these metals.
-
-
There are six possible isomers for a square planar palladium(II) complex that contains two Cl- and two SCN- ligands. Sketch the structures of all six, and label them cis or trans.
-
Carboplatin and platin are square planar complexes. Carboplatin can only exist as a single geometric isomer, but its relative platin has two geometric isomers.
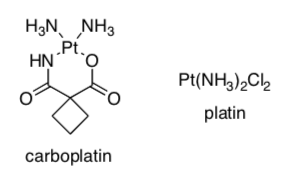
-
Draw both geometric isomers of platin and identify each isomer.
-
Explain why carbonplatin can only adopt one of the isomeric forms. possibible
-
-
Modeling How Nitrogenase Works
Nitrogenases are enzymes found in some soil-dwelling bacteria. These enzymes convert atmospheric N2 into NH3, which can be used by plants to make proteins, nucleic acids and other metabolites.
-
Draw a Lewis structure of N2.
-
What bond must be broken in order to make ammonia, and why is that difficult?
-
What is the bond order from your Lewis structure?
Nitrogenases contain transition metals such as molybdenum. An important goal of inorganic chemistry is to learn how metalloenzymes work.
-
What is the atomic electron configuration of Molybdenum atom (use noble gas abbreviation)?
-
Researchers recently reported a synthetic molybdenum complex (below) that can easily break the bond in .dinitrogen (Cummins, J. Am. Chem. Soc.2008, 130, 9394-9405).
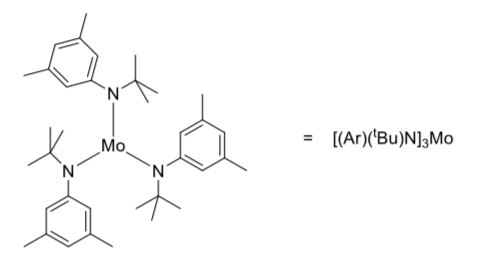
-
Fill in any missing electrons on N; it has an octet.
-
How many electrons does the molybdenum have in its valence shell in this molecules? (Mo's nonbonding electrons are not shown , but there is no formal charge.)
-
Are the valance shells of molybdenum satisfied?
-
What is the coordination number for Mo?
-
An N2 can bond with this complex by donating a pair of electrons. Show the new compound that results.
-
Now what is the new geometry at molybdenum?
-
Electron donation, in molecular orbital terms, can be thought of as the combination of a filled orbital on one atom with an empty orbital on another. For example, suppose the nitrogen donates a pair of "2s"-like electrons into an empty orbital on molybdenum. What are the next available empty orbitals for molybdenum?
-
In addition, electrons on the molybdenum can be given back to the N2. Show how the following d orbital could easily interact with an empty molecular orbital on nitrogen. How does that affect bonding in the N2?
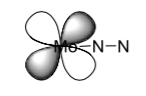
-
In similar way, a second molybdenum can bond to the order in the N2. Show the resulting compound.
-
Exposing the compound to ultraviolet light completely breaks the nitrogen-nitrongen bond, forming a molybdenum nitride (below). Explain why UV light can weaken the bond
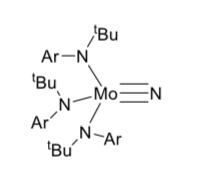
-
How many electrons does Mo have in its valence shell in the nitride complex?
-

