14: IMF
- Page ID
- 142564
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction to Dipoles
Electronegativity
-
Review: Define electronegativity:
-
As you move across the period from left to right electronegativity (increases/decreases or stays the same)?
-
As you move down a group electronegativity (increases/decreases/stays the same)?
-
What is the most electronegative atom?
Bond Dipoles and Dipole Moments
Polar bonds form between atoms of different electronegativity. This is described as a bond dipole and is represented using an arrow to indicate the direction of
electron displacement. 
-
Draw in the partial charges [(d-) and (d+)] on both NF3 and NH3.
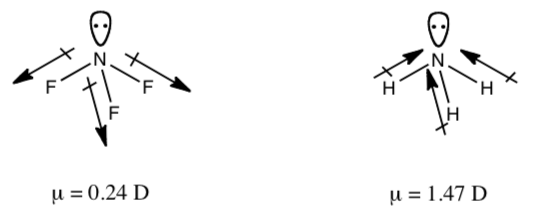
-
Draw the molecule H2O, with its correct geometry, and show the bond dipoles and partial charges.
Dipole Moments
The polarity of a molecule is due to the net sum of individual bond polarities. The net sum of dipoles is the dipole moment (μ).
For example, carbon dioxide has zero dipole moments even though both substances contain individual polar covalent bonds.
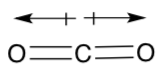
The individual bond polarities exactly cancel.
-
Draw both CBr4 and CH3Cl with the correct molecular geometry.
-
Draw in the dipoles on both CBr4 and CH3Cl.
-
One of these compounds has a μ = 0. Which one? Explain the difference in dipole moments in these two compounds.
Induced Dipole Moments
An induced dipole is a weak attraction that results when the electrons are disturb in a nonpolar species creating an instantaneous dipole. The electrons typically re-center and the dipole disappears.

-
What functional groups/molecules would have induced dipoles (keep in mind that they usually have no dipole)?
Practice Dipoles
-
Complete the following table to decide if the molecular contains polar bonds and/or if the molecule has a net dipole moment.
-
A periodic table with electronegativity values is available on the textbook site (a link is available in Sapling).
| Molecule | perspective Drawing with Dipoles | Molecular Geometry | Bonds Polar? | Molecular Polar? |
| CCl4 | Y/N | Y/N | ||
| HF | Y/N | Y/N | ||
| CS2 | Y/N | Y/N | ||
| BF3 | Y/N | Y/N | ||
| Acetone (CH3COCH3) | Y/N | Y/N | ||
| CH3CH2CH2CH3 | Y/N | Y/N |
Additional Problems
- Complete the following table to decide if the molecular contains polar bonds and/or if the molecule itself is polar.
| Molecule | perspective Drawing with Dipoles | Molecular Geometry | Bonds Polar? | Molecular Polar? |
| CH3CH2CH2OH | Y/N | Y/N | ||
| Benzene (C6H6) | Y/N | Y/N | ||
| SF6 | Y/N | Y/N | ||
| CH2Cl2 | Y/N | Y/N | ||
| CH3CH2OCH2CH3 | Y/N | Y/N | ||
| PCl5 | Y/N | Y/N | ||
| CH3CN | Y/N | Y/N |
Intermolecular Forces
Intermolecular forces (IMF) are forces of attraction between neighboring particles (atoms, molecules or ions).
- Coulomb’s Law:
-
Partial or whole positive charges are attracted to partial or whole _______________ charges.
-
The larger the effective charge, the ___________(stronger/weaker) the attraction between two particles.
-
The closer the two charges, the ____________ (stronger/weaker) the attraction between two particles.
-
-
IMF are ( weak OR strong ) compared to the covalent bonds which keep a molecule together.
The physical properties of melting point, boiling point, vapor pressure, evaporation, viscosity, surface tension, and solubility are related to the strength of the intermolecular forces.
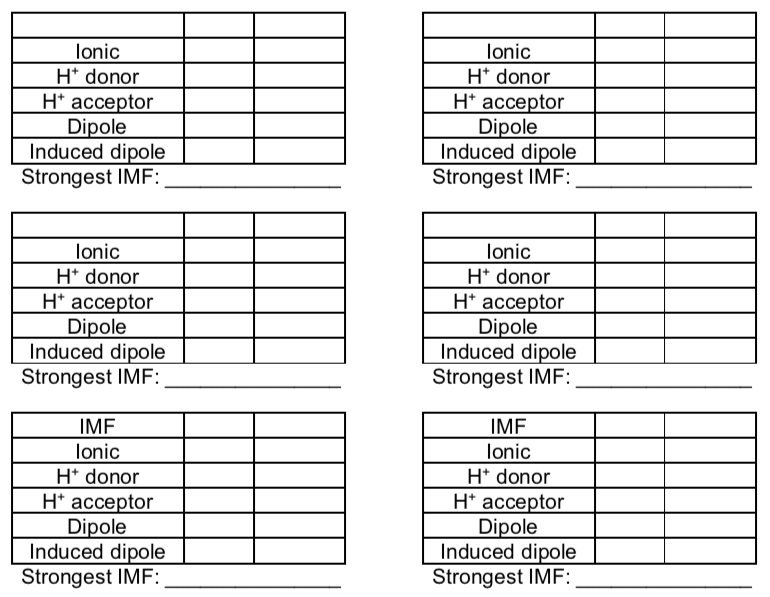
Intermolecular forces
-
Match the pictures of intermolecular interactions with the name.
ion-ion
dipole-dipole
ion-dipole
ion-induced dipole
dipole-induced dipole
London dispersion (induced dipole-induced dipole)
Hydrogen Bonding
A hydrogen bond is a type of intermolecular attraction. It is a particularly strong dipole-dipole interaction.
To have a hydrogen bond IMF, the molecule must have BOTH a hydrogen bond donor and a hydrogen bond acceptor.
A hydrogen bond donor is an H bonded to an O, N, F, or S.
A hydrogen bond acceptor is an N, O, F, or S atom with a lone pair.
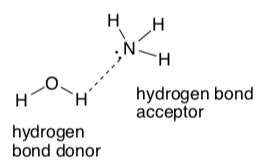
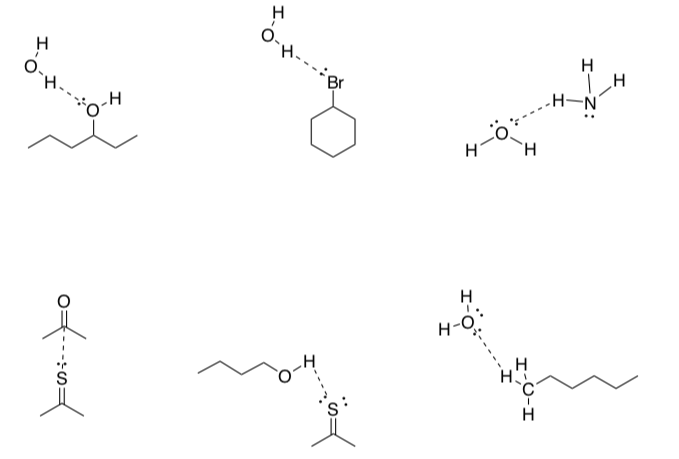
-
Circle which of following intermolecular interactions are hydrogen bonds.
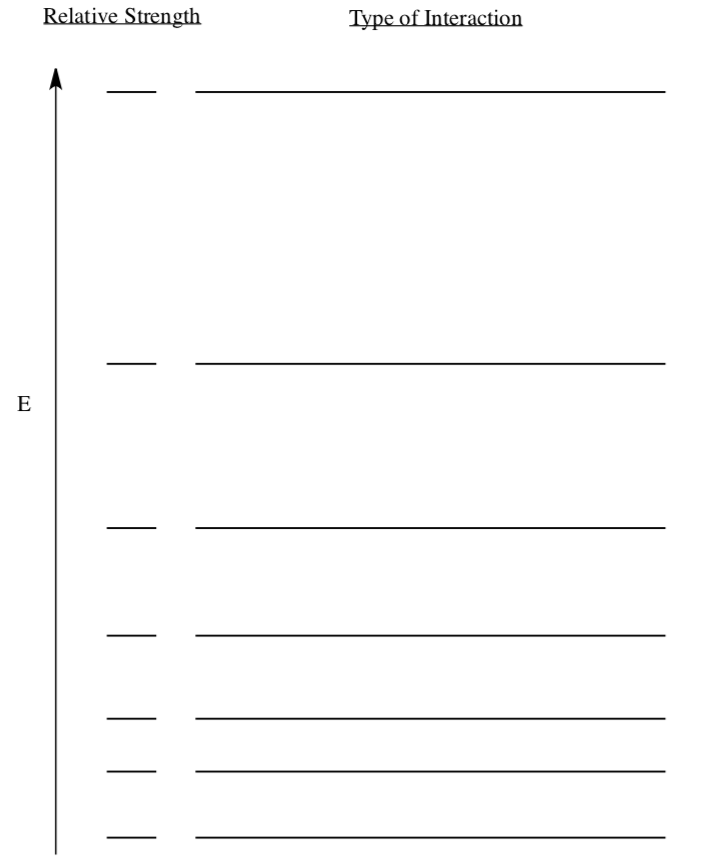
Strength of Intermolecular Attractions
- Rank the following IMFs by strength of coulombic attraction: ion-ion, London dispersion, dipole-dipole, ion-dipole, hydrogen-bonding, ion- induced dipole, induced dipole-dipole.
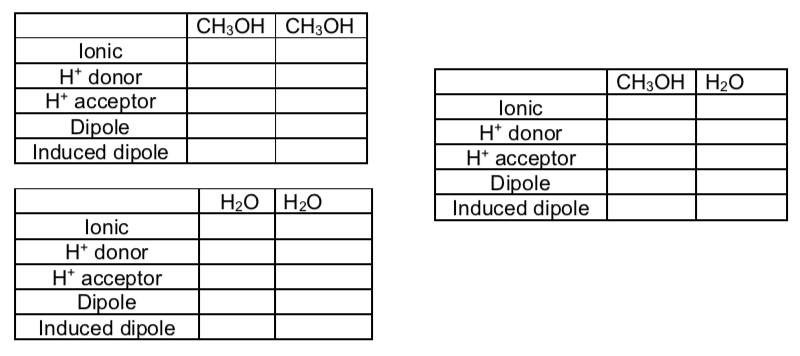
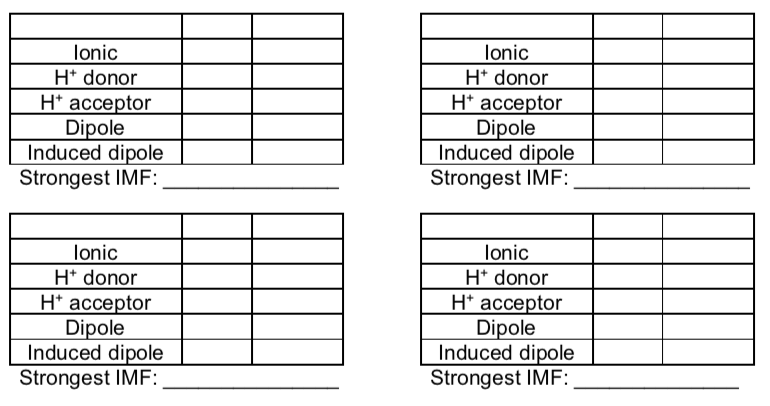
Intermolecular Interaction Guide
The tables below can be used to determine all interaction between two substances.
Note
H-bonding requires both a H+ donor and H+ acceptor.
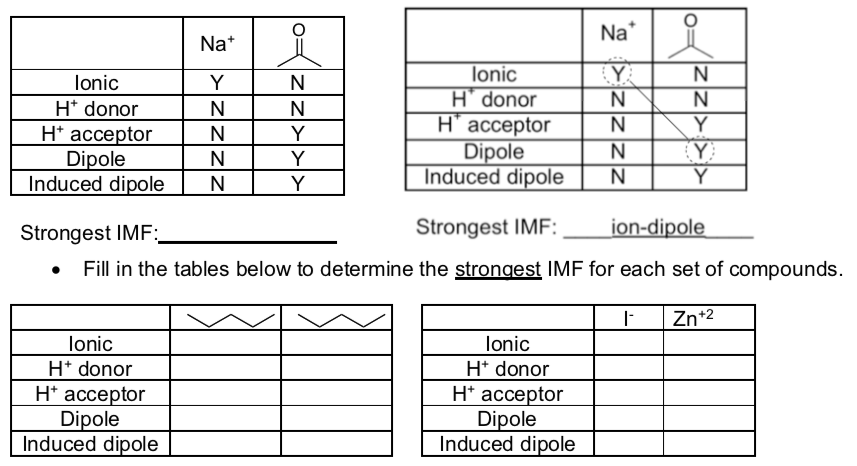
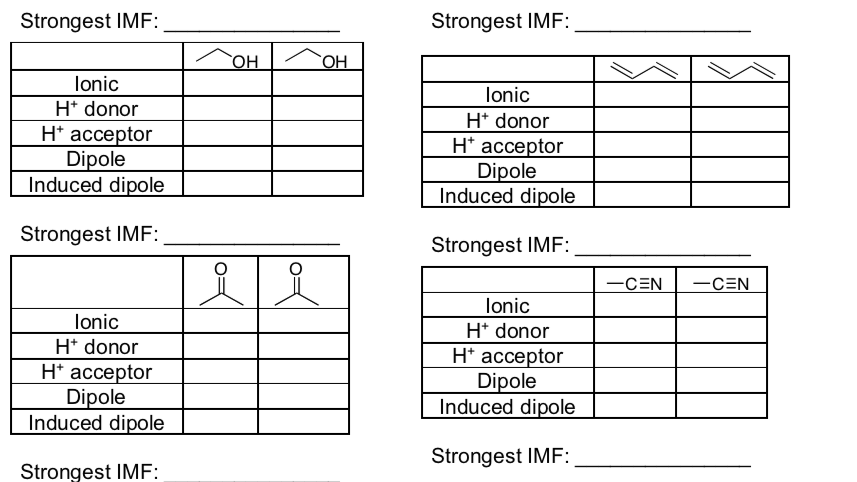
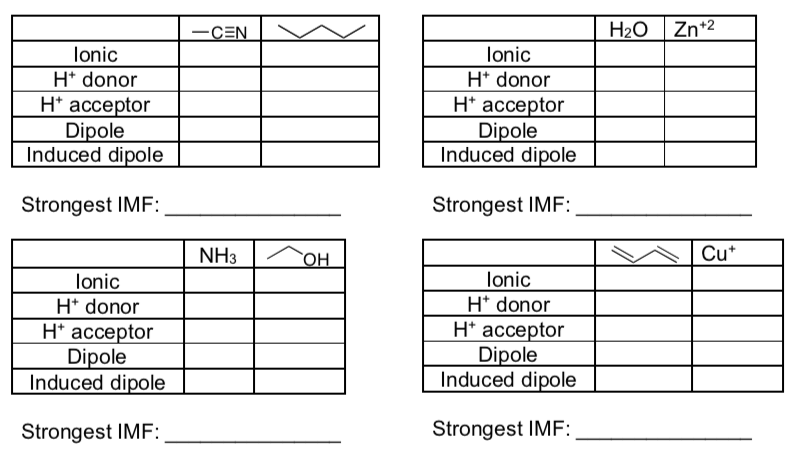
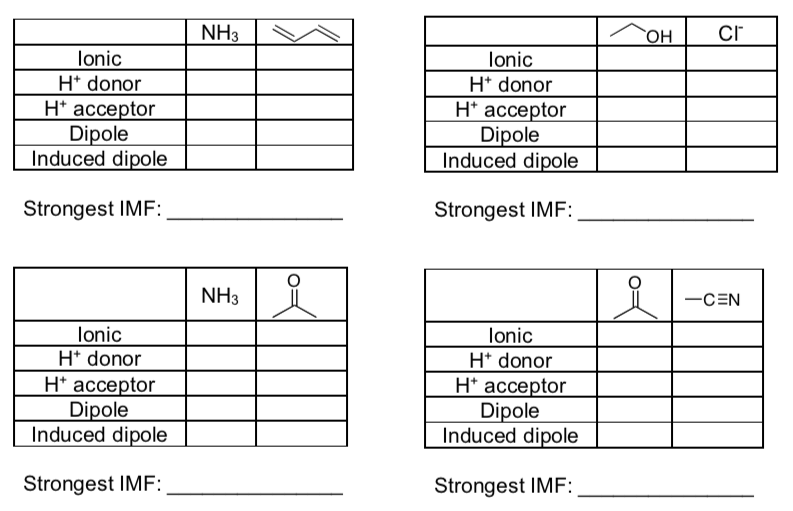
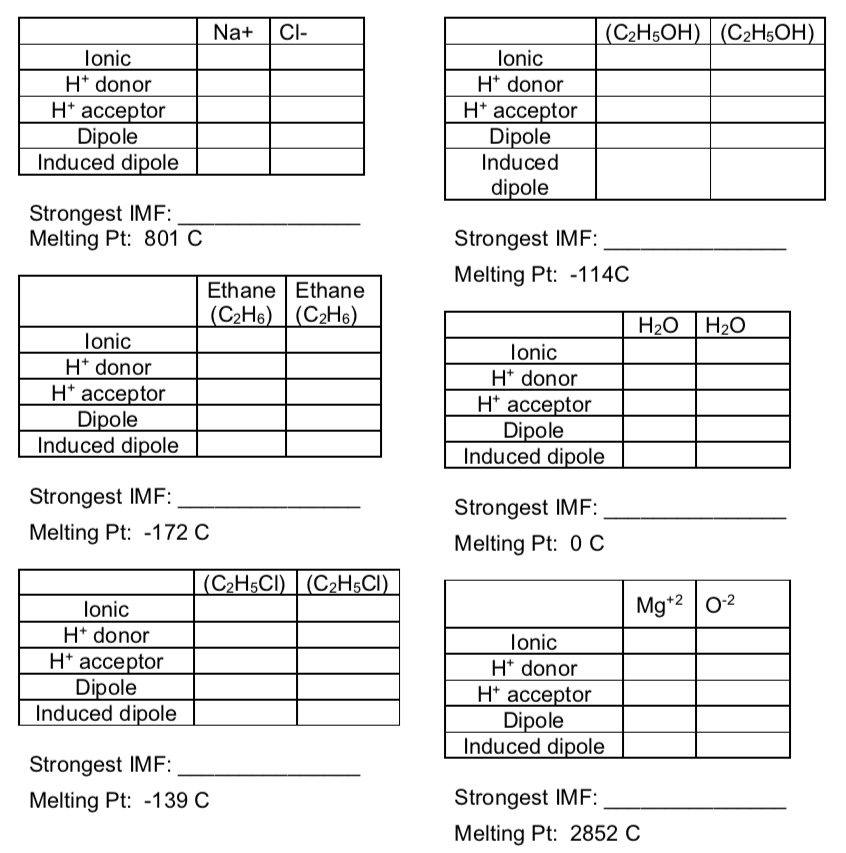
IMF in Mixtures
- Fill in the tables below and determine what the strongest IMF is for each set of compounds.
Properties of Molecular Compounds: Intermolecular Forces
IMF and Melting Points
Review: As the lattice energy increases, the melting point:
Decreases or Increases
- Compare the strength of the IMF in these compounds to the melting point.
Packing of Molecular Solids
Molecular compounds pack into repeating structures like metals and ionic lattices. Water forms a hexagonal molecular solid (ice) shown below. The open circles are oxygen; the shaded ones are hydrogen.
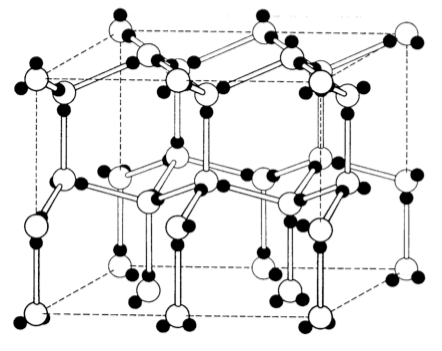
- What is the primary intermolecular force is holding this structure together?
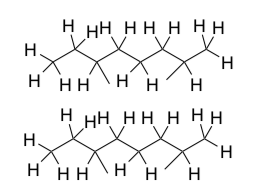
Paraffin Waxes are long chain alkanes (between C25-C40) from petroleum. Paraffin is a crystalline solid used for candle-making and glide wax on skis and snowboards.
- What intermolecular force(s) is holding this structure together?
IMF and Phase Changes:
The diagram below shows the temperature of H2O as heat is added.
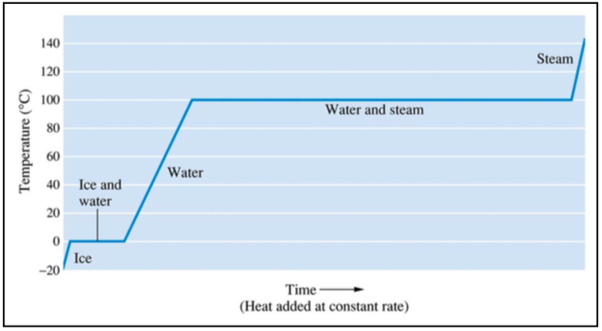
- Draw a cartoon of what is happening at the different temperature ranges. Show relevant IMF.
-
Between -20° and 0°.
-
Between 0°C and 100°C.
-
Between 100°C and 140°C
-
-
The graph has plateaus at 0°C and 100°C; energy is being added but the temperature is not increasing. Explain what is happening.
-
Is there a difference between melting point and freezing point?
IMF and Impurities in Melting Points
Water forms a hexagonal molecular solid (ice) held together with hydrogen-bonding. The open circles are oxygen; the shaded ones are hydrogen.
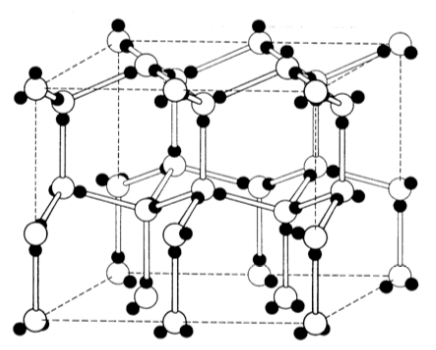
A structure that is less tightly packed will melt at a lower temperature (ie it requires less energy to break the lattice).
-
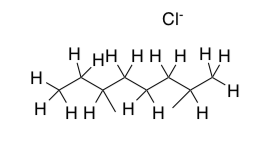
Na+ ions and Cl- ions, are placed in the holes of the lattice of the molecular solid, will the crystal be:
more tightly packed than pure ice?
the same as pure ice?
less tightly packed than pure ice (ie melt)?
-
A solution that has impurities (such as NaCl) dissolved in it, will
readily pack into a crystal (ie freeze)?
be less likely to pack into a crystal?
-
What happens when you add salt to your icy sidewalk in the winter? Explain in terms of crystal structures.
-
What would happen to the freezing point as the number of solute particles (dissolved particle or ions) in the solution increases?
-
CaCl2 is a more effective de-icer than sodium chloride. CaCl2 is effective in melting ice down to -25°F, whereas NaCl loses its effectiveness at about 20°F and is not useful for this purpose below 15°F. Why is CaCl2 more effective?
IMF and Boiling Point
Use the data from the previous page to answer the questions that follow.
-
Classify the functional groups below as polar or non-polar.
Class of Molecules Functional Group Bonds Non-Polar or Polar Alkenes Ketones C=O Alcohols -
Describe the type of intermolecular force (London Dispersion, Dipole- Dipole or Hydrogen Bond) possible in alkanes, ketones and alcohols.
Alkanes: Alkanes _____________________
Ketones: Ketones_____________________
Alcohols: Alcohols _____________________
-
Based on the strength of the IMF, rank of the boiling points of these functional groups (where 1 is the lowest bp and 3 is the highest bp).
Alkanes Ketones Alcohols
_____ _____ _____
-
For a given class of compound (alkane, ketone, or alcohol): How does boiling point change as the size of the molecule increases? Hint: Think about Velcro. Is it easier to pull apart a short piece of Velcro or a long strip of Velcro?
-
The boiling point of decane is 174 C but the boiling point of methanol is only 65 C. Explain why a molecule with only induced dipole-induced dipole interactions has a higher boiling point than a molecule with hydrogen bonding – a stronger IMF.
IMF and Vapor Pressure
When a substance boils, small bubbles rise to the surface and leave the liquid. These bubbles contain vaporized liquid molecules that have acquired enough energy to enter the vapor phase.
- Draw a cartoon of this process in the beaker.
The vapor pressure of a liquid is the pressure created by any molecules that leave the liquid phase and enter the gas phase.
If the external pressure (the atmospheric pressure in an open container) is greater than the vapor pressure, it will push molecules back into the liquid phase.
 If the pressure inside the bubbles is the same or greater than the external pressure, boiling will occur.
If the pressure inside the bubbles is the same or greater than the external pressure, boiling will occur.
-
A high vapor pressure means:
Lots of the molecules are in the vapor (gas) phase
Not many of the molecules are in the vapor phase
-
Molecules with low boiling points typically have:
high vapor pressures
low vapor pressures
-
Explain what happens to vapor pressure as a liquid is heated.
-
Assume that a liquid has a very high vapor pressure. Would you expect the intermolecular forces between the molecules between the liquid molecules to be strong or weak? Explain.
Vapor Pressure and Boiling Point with Ionic Solutions
When an ionic compound dissolves in water the cations and anions are separated and surrounded by water molecules.
NaCl(s) + H2O(l) → Na+(aq) + Cl-(aq)
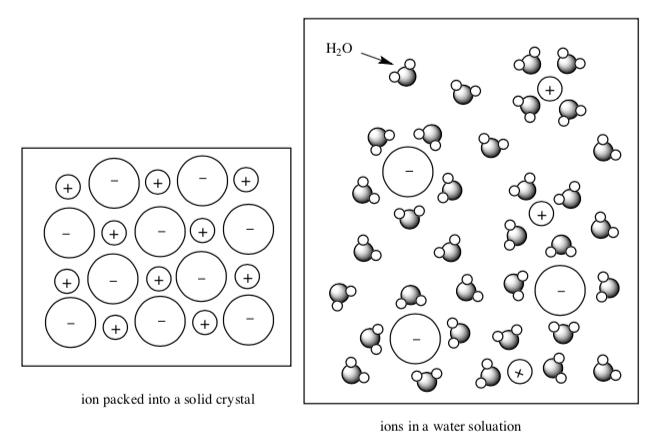
- Which is stronger?
-
Will the water molecules be held more tightly or less tightly in this salt water?
-
How will the strength of these interactions affect the number of molecules that escape the liquid into the vapor phase?
-
Complete the following statement: In general, the presence of a solute __________________ (increases/decreases) the vapor pressure of a solvent.
-
How will the number of dissolved ions affect the vapor pressure of a solvent? In general, as the number of dissolved ions increases, the vapor pressure _______________________
-
Complete the following statement: In general, the presence of a solute _________________ (increases/decreases) the boiling point of a solvent.
IMF and Surface Tension
Intermolecular forces attract molecules in liquids to each other. The picture below illustrates the intermolecular forces (arrows) in the body of the liquid and at the surface.
-
How many IMF attractions would a molecule on the surface feel?
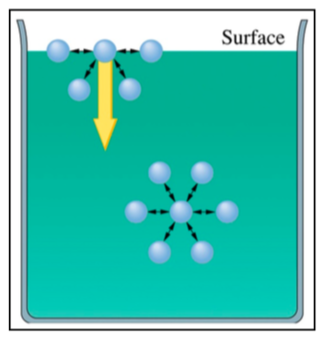
-
How many does the molecule feel in the body of the liquid?
-
In what direction are the molecules at thesurface being pulled in the figure shown?
-
Would this maximize or minimize the surface area? (Hint: spheres have less surface area than cubes)
Surface tension is the resistance of a liquid to spreading out and increasing its surface area.
- Complete the following statement: as the intermolecular forces in a liquid increase, the surface tension of the liquid_________________________.
IMF and Viscosity
Viscosity is also related to the strength of intermolecular forces. Viscosity is defined to be a resistance to flow (like t maple syrup compared to water).
-
How would the strength of intermolecular forces affect viscosity? Stronger IMFS would cause __________________________ viscosity.
-
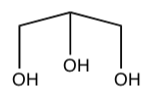
Which substance would have the higher viscosity: water or glycerol (shown below)? Why?
-
Which would have a lower viscosity, water or pentane (C5H12)? Why?
IMF and Solubility/Miscibility
A solution is a homogeneous mixture of a solvent, the substance present in excess, and the solute, the substance that is dissolved in the solvent.
Two liquids that completely dissolve in each other are miscible.
-
List the intermolecular forces involved.
-
Based on the IMF of the solute-solvent interactions, which are likely to make soluble combinations?
The strength of the solute-solvent IMF should be greater than or equal to the solute-solute or solvent-solvent interactions.
-
CH3OH in water.
Solute-solute interactions: ___________________________________
Solvent-solvent interactions: _________________________________
Solute-solvent interactions: _________________________________
-
-
Is this mixture miscible (soluble)?: YES NO
-
Hexane(C6H14) in water
Solute-solute interactions: ___________________________________
Solvent-solvent interactions: _________________________________
Solute-solvent interactions: __________________________________
Is the mixture miscible (soluble)? YES NO
-
NaCl in water
Solute-solute interactions: ___________________________________
Solvent-solvent interactions: _________________________________
Solute-solvent interactions: __________________________________
Is the mixture miscible (soluble)? YES NO
-
NaCl in benzene (C6H6)
Solute-solute interactions: ___________________________________
Solvent-solvent interactions: _________________________________
Solute-solvent interactions: __________________________________
Is the mixture miscible (soluble)? YES NO
-
Hexaneinbenzene
Solute-solute interactions: ___________________________________
Solvent-solvent interactions: _________________________________
Solute-solvent interactions: __________________________________
Is the mixture miscible (soluble)? YES NO
-
Here are blank tables for you to use:
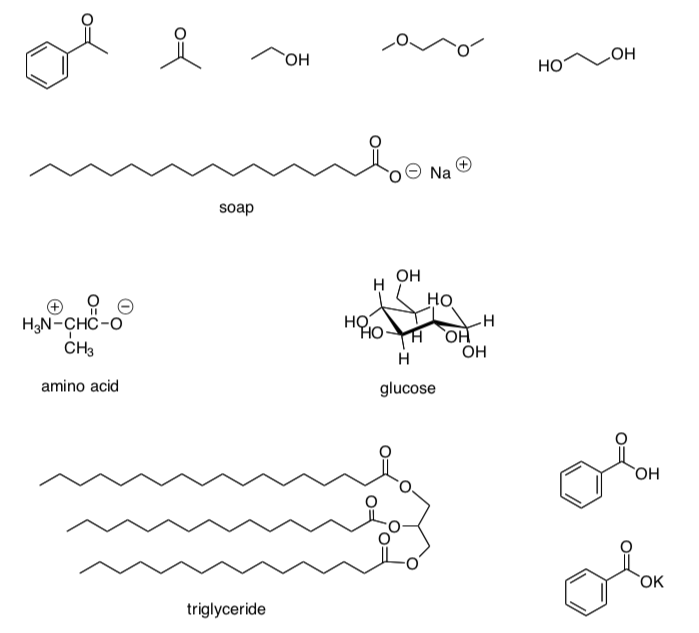
Guidelines for solubility of organic compounds in organic solvents vs water.
Organic compounds dissolve in organic solvents.
Organic compounds will ONLY dissolve in water IF:
-
the compound is charged
OR
-
the number of strong dipoles 3 the number of carbons.
- Put a box around any water-soluble compounds.
-
(Assume all other compounds would be soluble in organic solvents)
-
Summary
-
List the types of Intermolecular Forces.
-
Rank the IMF by strength.
-
How do Intermolecular Forces affect:
Melting Point?
Boiling Point?
Vapor Pressure?
Viscosity?
Miscibility?
-
How do impurities/solutes affect:
Melting Point?
Boiling Point?
Vapor Pressure?
Viscosity?
Miscibility?
Application Problems:
-
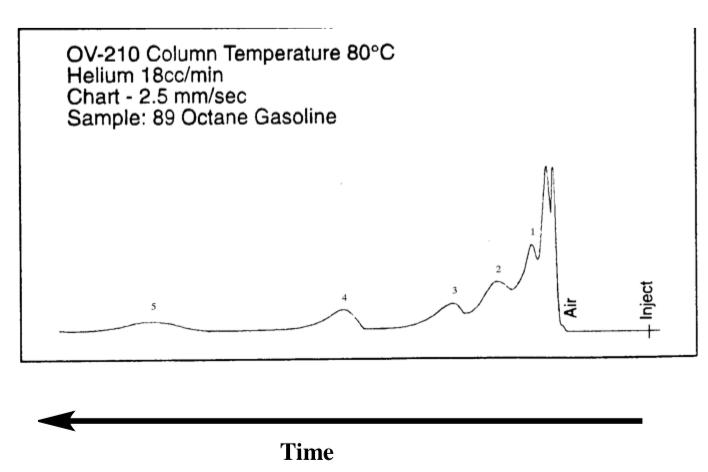
Gas chromatography separates mixtures by boiling point,with the lowest boiling liquid eluting first off of the column and the highest boiling liquid eluting last. A GC trace for a mixture is given below.
The mixture contains:
water
hexane (C6H14)
heptane (C7H16)
octane (C8H18)
ethanol (CH3CH2OH).
-
Identify the compound for each of the labeled peaks.
NB: The compounds start eluting (coming off the column) from right to left on this chromatogram.
Peak 1: ___________________ Peak 4: ___________________
Peak 2: ___________________ Peak 5: ___________________
Peak 3: ___________________
-
-
Ethylene glycol(HOCH2CH2OH)is commonly used in cars to prevent the water in the radiator from boiling over. Explain why this works.
-
The cleaner, Goof Off (the MIRACLE remover), is advertised to remove grease, oil and wax from surfaces. Goof Off is composed of a mixture of xylene and ethyl benzene. The structures of these compounds is shown below:
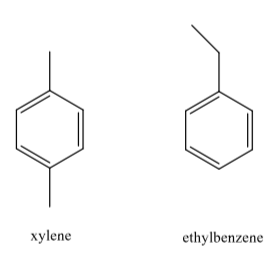
Grease, oil and wax are hydrocarbon compounds. Why does Goof Off work better than water to remove these substances?
-
Performance Fabrics
Performance fabrics are popular in athletics because they let the athlete feel dryer even when working hard. Performance fabrics are generally polyesters. Traditional fabrics are most commonly made from cellulose fibers derived from cotton. Portions of these very large molecules are shown below.
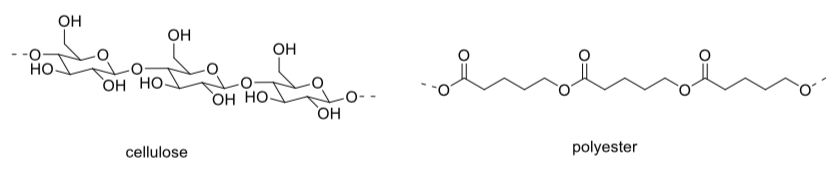
Explain, with the help of drawings, why water can pass through performance fabrics and easily evaporate, whereas cotton becomes soggy during a workout.
-
Soap
An example of a soap molecule is sodium stearate (pictured below).
-
What types of intermolecular forces occur between soap and water?
-
What types of intermolecular forces occur between soap and grease (hydrocarbons)?
-
Describe how soap works. (draw a picture)
-
In “hard water”, there are Ca+2 ions. If the calcium ions replace the sodium ions, what will the soap look like?
-
Calcium salts of most soaps are not soluble in water and these salts precipitate out of solution as “scum” or “bathtub ring”. How will this affect the effectiveness of your soap?
-
-
Petroleum Wax in Pipelines
Crude Petroleum is made up of several types of hydrocarbons.
When hot Petroleum oil is flowing in a pipe in the ocean, wax build-up is a big issue. This wax deposit causes problems in oil production and transportation (see picture below)
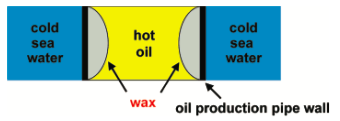
Gas chromatography was performed on the wax deposit in pipelines to determine what types of waxes were present.
Several polymers known as commercial flow improvers and wax inhibitors have been used to add to prevent the wax deposit. Recently, researchers at Cal Tech attempted to model this system to develop better wax inhibitors. Jang, Blanco, Creek, Tang and Goddard, J. Phys. Chem. B, 2007, 111, 13173-13170.
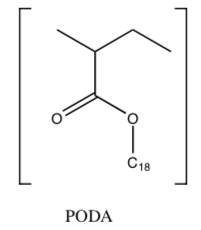
This group used a polymer of poly(octadecyl acrylate) (PODA) as the model inhibitor (shown below).
A decamer (a polymer of ten repeating units) of syndiotactic PODA (PAA1, MW 3276) was chosen as a model inhibitor representing the PODA. When n by Comb-like Polymer oil, the amount of wax deposit is reduced from 72 g/day to 13.69 g/day.
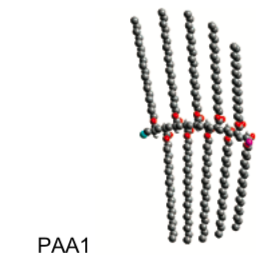
-
Circle the physical state of the compounds shown below at room temperature:
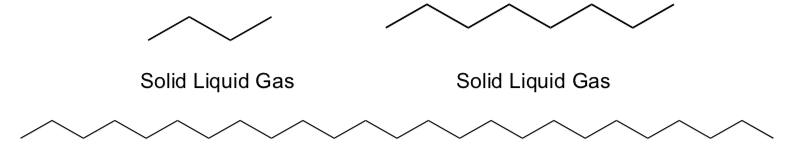
-
Explain your choice.
-
Explain why this is happening in terms of physical states.
-
Describe what IMF are involved in the wax deposition.
- In the GC shown below, how many alkanes are present in the wax?
- Which alkanes are most prevent?
-
What is the main functional group?
-
Why does PAA1 reduce was deposit? Explain using your understand of IMF and packing of molecular solids.
-
Would a polymer of poly(hexyl acrylate) be more or less efdfecftiinve?d aWs h1y?fo
-
-
How do you remediate a massive oil spill?
An explosion on an oil rig on April 20, 2010 led to an oil spill nearly a mile deep in the Gulf of Mexico. This started an ecological catastrophe that will have long-term implications for ecosystems and the economy in the Gulf. Oil is a complex mixture of organic hydrocarbon molecules. In general it consists of straight or branched chain alkanes (CnH2n+2), cycloalkanes, and aromatic hydrocarbons (HC).
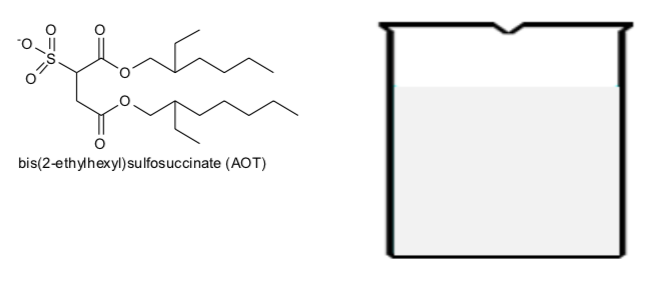
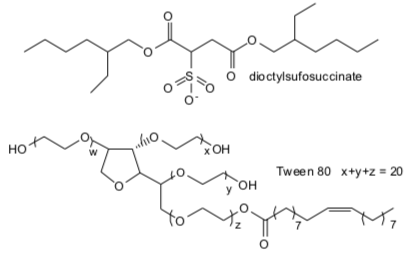
Now consider a slightly different scenario. Add a small amount of sodium bis(2- ethylhexyl) sulfosuccinate (AOT) and small amount of water to a great excess of a hydrocarbon solvent, octane.
BP has injected enormous amounts (up to 1.5 million gallons) of the dioctylsulfosuccinate (DOSS) and Tween 80 (shown below) into the oil leak, which is releasing about 80,000 barrels a day.
Recent analyses suggest that a lot of oil from the Deepwater Horizon didn't simply evaporate or dissipate into the water — it has settled to the seafloor.
-
Draw a few representative examples using your table of functional groups.
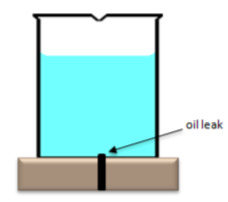
-
The volume of the oil spill is obviously much smaller than the volume of the Gulf into which it is spilling. Show in the beaker of water to the right what would happen to the oil if a small amount of oil relative to the volume of pure water was injected at the site shown into still water.
-
Assuming no more injection of oil, what would eventually happen to the oil if left for months? How would your answers differ if the water was mixing vigorously as the oil was injected?
-
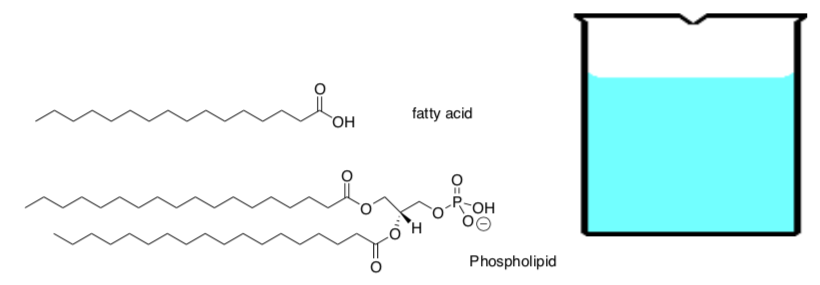
How do fatty acids and phospholipids (example of which are shown below) interact with self and water? Draw the lipids in the beaker below in “cartoon” form as either single chain or double chain amphiphiles (molecules with discreet polar and nonpolar sections).
-
Draw a cartoon form of AOT in the beaker of hexane below and show how it interacts with water and octane. Compare your diagrams with those for fatty acids and phosphollpids in excess water.
-
Draw a cartoon showing the interaction of the substances with oil. What are the main differences between the DOSS and Tween 80?
-
What effect do you think the oil will have on biological life deep in the ocean?
-
-
Recrystallization
Recrystallization is a common lab purification technique. It relies on solubility and lattice energies.
Assume that the impurity is very soluble in water but the crystal compound is only slightly soluble.
As the sample cools, the crystal compound wants to reform as a solid because it isn’t very soluble. The impurity stays in solution.
Overall, recrystallization is an inefficient purification method as you only get approximately 70% of your crystalline solid reformed.
-
Draw a picture of a solid crystal with an impurity.
-
Draw a picture of the impure solid dissolved in water.
-
Draw a picture of the system after cooling.
-
Why?
-
If you use too much water as the solvent, what will happen?
-
-
HEET
Often, a pint of HEET is added to the gas tanks of cars, especially in the coldest days of winter, to prevent gasline freeze-up. (Small amounts of water in gasoline can freeze when it is very cold. This blocks the fuel line and stops the engine.)
-
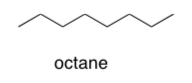
Assume that gasoline is pure octane (C8H18). What sort of forces will hold the octane molecules to each other in gasoline?
-
Explain and draw a picture.
-
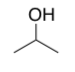
One type of HEET contains almost 100% isopropyl alcohol, shown below. Show how isopropyl alcohol molecules will interact with each other. What kinds of forces hold them together?
-
Isopropyl alcohol is completely soluble in both water and gasoline. Why? Draw two pictures of isopropyl alcohol to explain:
a) interacting with water b) Interacting with octane
-
When HEET is added to a tank of gas it tends to cause water to dissolve (or at least stay suspended) in gasoline. Show in a picture and explain how this happens, on the molecular level.
-
All gasoline in Minnesota is required to contain 10% ethanol (CH3CH2OH). Given this formulation, do you need to use HEET? Explain.
-
-
Although tert-butyl methyl ether (TBME) and water initially appear to be immiscible, a small fraction of ether will actually dissolve in water and vice versa. This has been reported as a problem recently when TBME, sometimes used as a gasoline additive, has leaked out of underground gasoline storage tanks and contaminated groundwater, resulting in unusually flavoured drinking water.
-
Draw a structure of TBME, (CH3)3COCH3.
-
Use drawings to illustrate intermolecular forces in the following places:
-
a glass of water
-
a glass of TBME
-
a glass of water containing some TBME
-
-
Explain briefly why TBME is very slightly soluble in water. What is it that makes a little bit of it dissolve? What keeps more of it from dissolving completely?
-
-
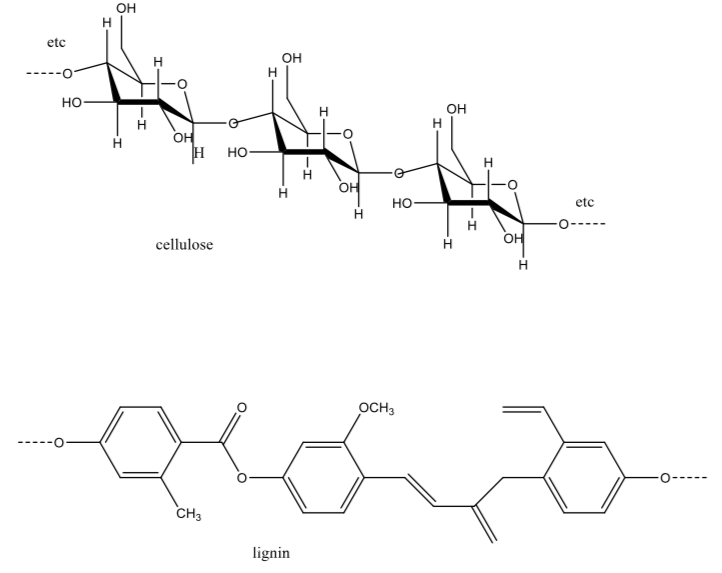
Paper is made from wood. Wood is composed mostly of cellulose and lignin, long molecules of which sections are shown below. To make stronger paper, the lignin is separated from the cellulose.
-
Explain with words and drawings what holds the cellulose together in the paper.
-
Why does removal of the lignin make the paper stronger (i.e. it is harder to rip if there is no lignin)?
-
Paper still gets soggy and falls apart if it gets wet. Explain why with the help of words and drawings.
Another problem is that water-based inks are absorbed too heavily into the paper, making the ink blot. To offset these problems, sizing is added to the surface of the paper. One common material used for sizing is abietic acid (below).
-
Explain with words and drawings how sizing adheres to the paper, and how it alters the water-absorption properties of the paper's surface.
Paper is somewhat transparent. To make it opaque and white, salts such as titanium oxide (TiO2) are added to the cellulose before it is rolled into sheets.
-
Why does TiO2 adhere to the paper?
-

