7.4: London Attractions
- Page ID
- 138867
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)All molecules can experience London interactions or dispersion forces (sometimes called van der Waals interactions, although many people use this term to describe any kind of intermolecular attraction). These interactions are temporary electrostatic attractions caused by the random movement of electrons around a molecule. If at one moment more of the electrons are found on one side of the molecule than the other, then the molecule will develop a dipole.
A dipole is a separation of charge. If electrons slosh over to one side of the molecule, that end of the molecule becomes negatively charged, because electrons are negative. The other end of the molecule, however, becomes positively charged, because the negatively charged electrons that should be balancing the positive charge of the nucleus have drifted to the other end of the molecule. We symbolize this charge separation with a dipole arrow. One end of the arrow looks like a plus sign; that's the more positive end. The pointy end of the arrow represents the negative end.
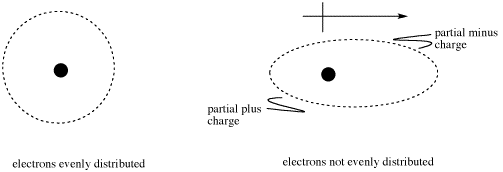
This dipole is transient, meaning it does not last very long. However, in its short lifetime it could actually induce another dipole in a neighboring molecule. The electrons gathering on one side of one molecule could repel the electrons on the near side of the next molecule. This situation would leave the more negative end of one molecule next to the more positive end of its neighbor, and a weak attraction would result.
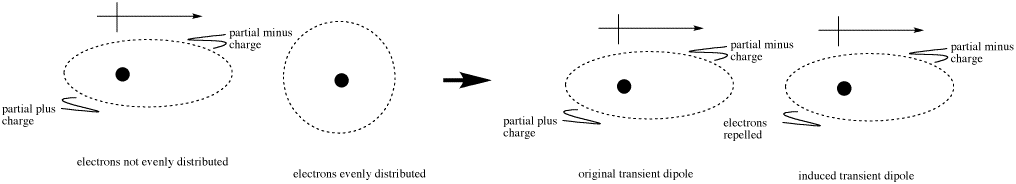
In most molecules, and especially relatively non-polar ones, these kinds of interactions last for tiny fractions of seconds. That means that if molecules rely entirely on London interactions to stick together, they probably don't stick together very well.
However, there are a couple of factors that can make these small interactions add up until they are pretty significant. First, molecules with many, loosely-held electrons would experience more London interactions than molecules with fewer, more tightly-held electrons. That's because the more electrons there are, and the more freely they are moving, the more likely they are to create that initial transient dipole. This factor is pretty significant when comparing the halogens, since fluorine has nine tightly-held electrons while iodine has fifty three, and most of them are so far from the nucleus that they are hard to keep track of.
In addition, this type of attraction does not work very well between two molecules that are far away from each other. Because it is such a weak attraction, it works best when the two molecules are very close to each other. The more contact there is between the surfaces of the two molecules, the more likely it is that one molecule can induce a dipole in its neighbor, and the stronger the attraction will be.
It's all a matter of surface area. The more surface contact between two molecules or two materials, the more likely they are to interact with each other. In fact, this sort of phenomenon can lead to some surprising results if taken to the extreme. Many researchers believe that the ability of geckos to walk on walls and ceilings, even if the surface is very smooth, is entirely due to van der Waals forces. Millions of tiny fibers (or setae) cover the bottoms of the lizard's feet, and each of these fibers is covered in hundreds of tinier pads (or spatulae). These structures ensure that there is enough surface contact for many, many weak interactions to add up to one strong grip. Some companies are even trying to use this idea to make very strong but removable adhesives based on this gecko model.
Now consider the difference between the attractive forces that would be found in two different shapes of molecules. In one case, the molecules are small and round, like ping-pong balls. In the other case, the molecules are long, and linear, like ribbons of spaghetti or lasagna.
You can arrange ping pong balls in any way you like (without squashing them flat), and they will never be able to do more than barely touch their neighbors.
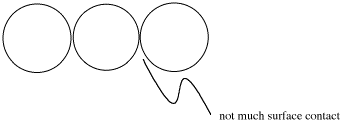
Lasagna noodles stack very easily on top of each other, and have a good deal of contact with the other lasagna noodles.
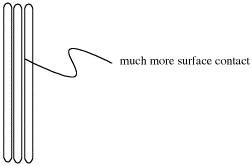
If London interactions rely on temporary dipoles that are induced only if molecules can get very close to each other; it would be best if the two molecules could almost touch each other.
This factor results from the physical law that electric forces fall off very quickly with increasing distance. The farther apart the molecules, the less strongly they can have an effect on each other.
So, the long, ribbon-like molecules, having more surface area in contact with their neighbors, are more likely to induce dipoles in their neighbors, and are more likely to stick together. The spherical molecules, with less surface area in contact, will attract each other more weakly.
What sort of molecules are shaped like ping pong balls and spaghetti? Two very similar molecules, both with the formula C5H12, can serve as examples.

|
|
Wire Frame Ball & Stick Spacefilling |
Normal pentane, or n-pentane, is a molecule in which all five carbons are arranged in a string, with the hydrogens completing the valence of each carbon. Neopentane, or 2,2-dimethylpropane, is a molecule in which one central carbon is bonded to each of the four other carbons, and each of those four carbons has three hydrogens on it. On paper, one might imagine pentane is spaghetti shaped, but neopentane looks more like a cross. You need to look at a space-filling model to see that neopentane is very much like a ball.
|
|
Wire Frame Ball & Stick Spacefilling |
It turns out that pentane has a boiling point of 35 oC, while neopentane, with the exact same molecular formula, has a boiling point of -9 oC. That means that, at comfortable room temperature, pentane is a liquid, while neopentane is a gas even on a pretty cold day. The pentane molecules stick together more because there is more contact between them; the neopentane molecules don't, and they each go their own way, forming a gas.
Pentane and neopentane are isomers. They have the same formula but different structures. Isomers is from the Greek, meaning "same things", referring to their identical content. However, isomers often have very different physical and biological properties.
Problem SP4.1
Compare London forces in the following pairs of molecules, and predict the relative melting points of the two compounds.
a) CH3(CH2)6CH3 and CH3(CH2)100CH3 b) CH3(CH2)6CH3 and CH3(CH2)3C(CH3)3 c) CH3Br and CH3Cl
Problem SP4.2.
Predict the relative boiling points of 3,4-dibromohexane and 3,3-dimethylhexane.
Problem SP4.3.
What would you predict about the relative melting points of dibenzyl ether and bis(cyclohexylmethyl) ether. (Note: "bis" is often used instead of "di" as a prefix for more complicated groups.)


