Sample Introduction in Gas Chromatography
- Page ID
- 283979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
All students will be able to:
- Describe the operational principle of different entry-level techniques such as split/splitless, on column, PTV, thermal desorption-pyrolysis, purge and trap
- Differentiate various types of GC sample introduction methods and determine their suitability for specific analytes in different matrices
- Interpret the impact of sample introduction on a GC column (particularly on a capillary system) and on the resulting chromatogram from a perspective of how resolution and response for splitless and split injection would be affected.
GC inlets Home to take Quiz
Provide handwritten answers and bring them to the class
- What types of inlets do you know for capillary GC?
- For which types of analytes matrices would you use these inlets?
- What are the major requirements on the inlet?
- Explain (sketch) how a split injection works. What are the typical split ratios and how they are related to the dimensions of a column and sample capacity?
- What is sample discrimination?
- What are the advantages/disadvantages of splitless injection?
- What is a suitable injection volume? How is it related to backflash?
Considering the narrow liner being 4 mm ID, 90 mm long, with an injection volume of 1 µL, head pressure of 5 psig and injection port temperature of 250 °C, what would be a good and a bad solvent for sample injection and why? Would hexane be suitable?
- What is the source of peak fronting, and how can you prevent it?
- How would you correctly select the splitless time (the time the purge valve opens)?
- What kind of problems would you expect to be caused by a septum?
- What is the retention gap? Why would you use it for an on-column injection?
- What is PTV? How does it work?
A. GC Inlets Types
You have a dream lab with several GCs with different inlets: split/splitless, on column, PTV, thermal desorption-pyrolysis, purge and trap.
- Which GC system would you use to analyze the following samples:
- Total petroleum hydrocarbons (TPHs) in gasoline
- Nitrated polycyclic aromatic hydrocarbons (N-PAHs) in air particulate matter
- Alcohol content in wine
- Chlorinated disinfectant products in water
- Polychlorinated biphenyls (PCBs) in soil
- Nanopolymeric materials
- Total petroleum hydrocarbons (TPHs) in gasoline
- Provide an explanation for your selection, consider if you need to perform an extraction or some other sample preparation.
- What concentrations of target analytes do you expect to find in these samples?
- How reactive and/or thermally stable are each of the targeted analytes?
- Considering your answers in 3 and 4, would you change anything in your answer to question 1? Why?
B. SPLIT Inlet in GC
Consider Split GC Inlet,
- Denote where in the diagram are the following items: the septum purge, split valve, liner, and what the direction of gas flow is
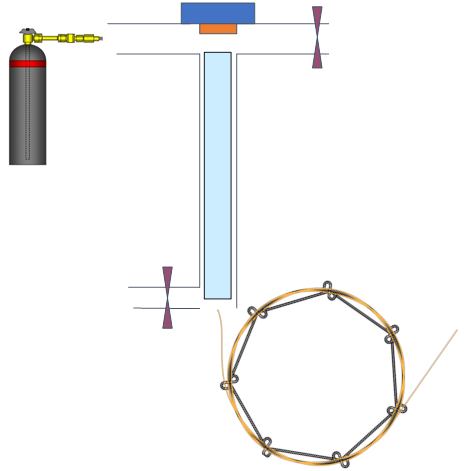
Note
Typical syringe needles are 4 cm long and the liner is about 5 cm long
The septum purge flowrate 3 mL/min
The split flowrate 50 mL/min
The column flowrate 1 mL/min
- What is the purpose of septum purge?
- What is the septum made of?
- Sketch an injection syringe needle in the injection port and consider how deep it goes.
- (revisit question 2 if needed)
- Sketch two chromatograms showing the elution of C6 to C18 alkanes, if they were injected at injector temperatures of 200 °C and 300 °C (assume that they will elute in the order of boiling points)
- Based on the carrier gas flow rates, determine the flowrate through the liner.
- Consider the time needed for vaporization in the inlet and how this will affect species of different volatility.
- Redraw chromatograms (question 6) considering points 7 and 8 with regard to possible discrimination.
C. Splitless Inlet in GC
Consider GC analysis of benzene (10 µg/mL) dissolved in hexane injecting 1 µL to a splitless inlet heated to 250 °C.
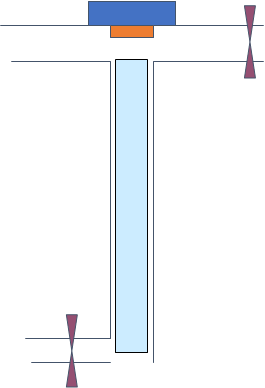
Note
Volume of the liner is 1 mL.
- Propose flowrates at the outlets and through liner when the split valve is closed assuming column flowrate of 1 mL/min.
- What would happen if you injected 5 µL of sample?
- Determine volume of gas phase when 1 µL of hexane is injected. (revisit question 2 if needed)
- Sketch chromatograms comparing what will happen if the split valve is closed for the period of entire analysis compared to the chromatogram with the splitless valve open after 1 minute.
D. Splitless time and solvent trapping
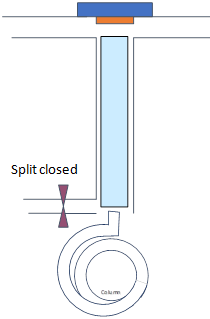
Consider splitless injection as above with an injector temperature of 250 °C and initial column temperature 100 °C for 1 min, split closed for 1 min (i.e., splitless time) and the flow rate on the column is 1 mL/min for a sample of benzene dissolved in hexane.
Regular splitless
|
Time 0-1 min |
Time 1-1.2 min min |
|---|---|
|
Splitless Time 1 min |
Splitless Time 1 min |
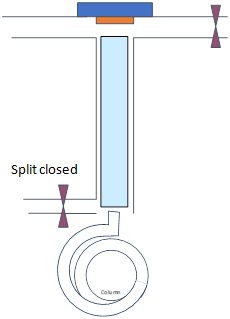 |
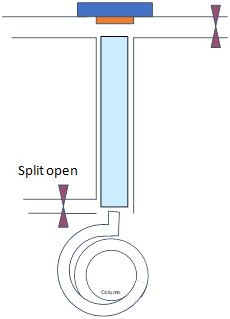 |
|
Tcol = 100 °C |
Tcol = 100 °C |
- Draw schematics of gas (dots) and liquid (droplets) phases of the analyte and solutions within the injector and the column for times specified on top row (note how they travel)
Consider the analyte boiling points
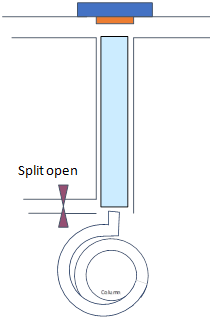
Consider a solvent trapping splitless injection with T of injector 250 °C, flow rate on the column is 1 mL/min for a sample of benzene dissolved in hexane.
|
Time 0-1 min |
Time 1-1.5 min |
Time 0.5-0.8 min |
|---|---|---|
|
Splitless Time 1 min |
Splitless Time 1 min |
Splitless Time 0.5 min |
 |
 |
 |
|
Tcol
|
Tcol
|
Tcol
|
- Draw gas (dots) and liquid (droplets) phase of analyte and solutions within the injector and the column for specified times and considering two different splitless times (note how they travel)
- What will be the initial temperature of the column to ensure the solvent trapping of hexane?
- What will be the next temperature step for the column?
- Considering these temperatures, revisit question 2.
- Draw three chromatograms for the sample injection of benzene dissolved in hexane
- without solvent trapping (see the previous page)
- with solvent trapping for a splitless time of 1 min.
- with solvent trapping for a splitless time of 0.5 min.
- Will the peak width change? Explain your answer.
- Will the retention time change?
- What else may change?
Contributors and Attributions
- Alena Kubatova, University of North Dakota (alena.kubatova@und.edu)
- Sourced from the Analytical Sciences Digital Library


