23.2: Derivatization
- Page ID
- 332828
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Absolute Configuration: Derivatization*
Chiral Derivatization Methods
A chiral derivatizing agent typically reacts with an alcohol or amine of unknown stereochemistry to form an ester or amide.
- Would 2(R)-pentanol and 2(S)-pentanol have different NMR spectra?
- Complete the following reaction scheme for the reaction to form Mosher ester A and Mosher ester B.
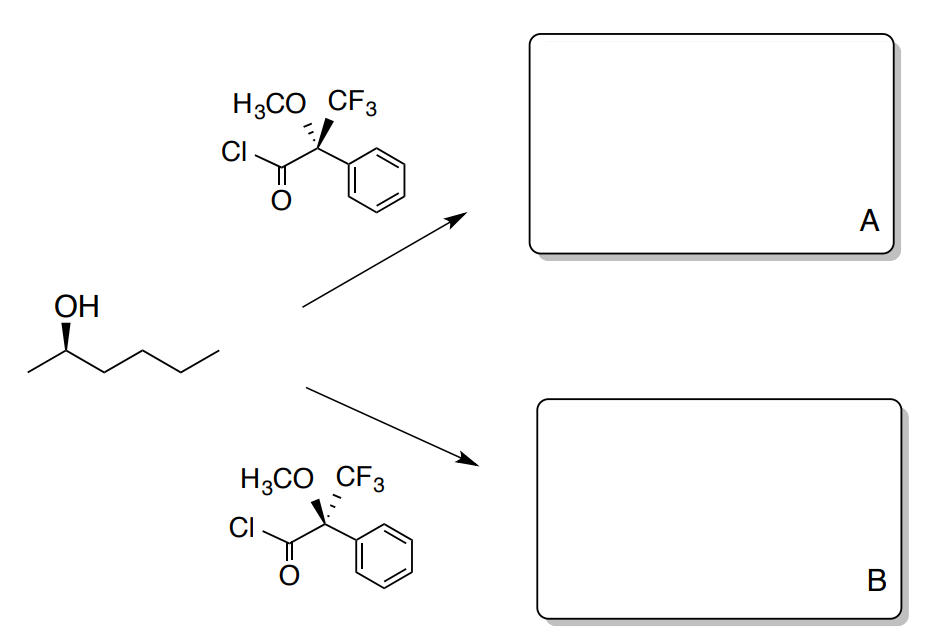
- Designate the stereochemistry of the starting alcohol, the two reagents and the two esters (A and B).
- What is the isomeric relationship between the two reagents?
- What is the isomeric relationship between the two products (A and B)?
- Would there be a difference in spectra of A and B?
* All spectra are either from SDBS (Japan National Institute of Advanced Industrial Science and Technology) or simulated.
Effect of Chiral Derivatives on NMR Shift
There are several chiral derivatives. Mosher esters are just one of several similar types of methods for derivatization.
Seco, Quinoa & Riguera, The Assignment of Absolute Configuration by NMR, Chem. Rev., 2004, 104, 17-118.
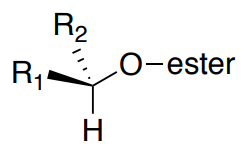
A critical part of the analysis of these derivatives is to determine the most stable conformation of the new ester.
- Usually, it is assumed that the H next to the secondary alcohol on the original structure will align with the carbonyl.
- Additionally, the trifluoromethyl group (or other electronegative group) will stay in the plane to avoid the LUMO of the carbonyl.
- This leaves one group on the original alcohol aligned with the phenyl (designated as R1) and one aligned with the methoxy of the ester (R2).
For the S ester derivative:
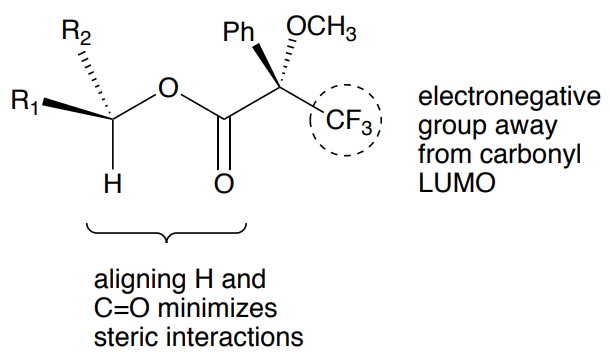
- The R1 group will be closer to the phenyl group, shielded and will shift upfield.
- The R2 group will be closer to the methoxy, deshielded, and will shift downfield.
- Draw the R ester in the most stable configuration.
- What will happen in the R ester derivative,
- To the R1 group shift?
- To the R2 group shift?
NMR of Mosher Esters
Usually, chemists will make both possible diastereomeric esters of the alcohol group. The 3D picture of the two chiral esters formed from 2(R)-pentanol are shown below.
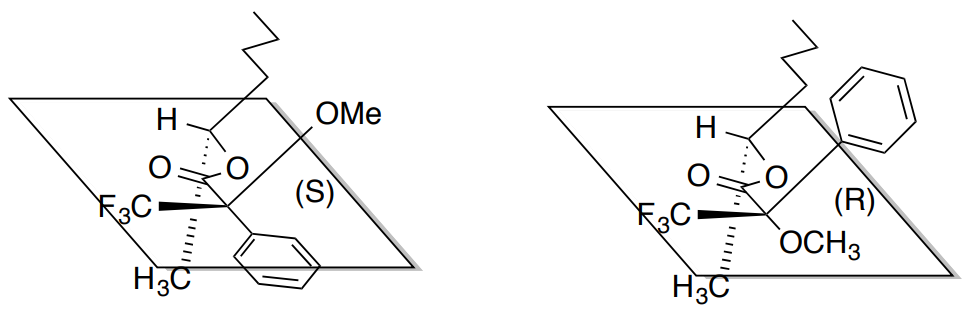
When analyzing the NMR spectra, there will be differences in chemical shifts for protons located near the stereocenter of the alcohol.
- Protons in the butyl group in the S-ester will be shifted [ downfield / upfield ] relative to the butyl group in the R-ester.
- Protons in the methyl group in the S-ester will be shifted [ downfield / upfield ] relative to the methyl group in the R-ester.
A. 1H NMR shift for the methyl group of 2-pentanol.
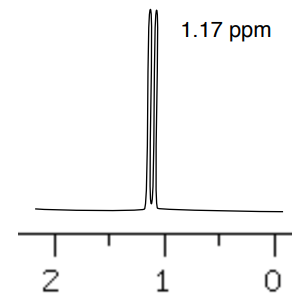
B. 1H NMR shift for the methyl group of the [ R or S ] ester derivative.

C. 1H NMR shift for the methyl group of the [ R or S ] ester derivative.

Modified Mosher Analysis
The R2 and R1 groups have slight shifts in the Mosher ester derivatives. The shifts are in the opposite direction for the R ester derivative and the S ester derivative. But, these shifts are often very small.
To emphasize the shift changes, the parameter, ∆δSR, which is defined as the difference between the chemical shift of a certain proton in the (S)-MTPA ester and the chemical shift of the same proton in the (R)-MTPA derivative.
Thus, the protons shielded in the (R)-MTPA will present a positive ∆δSR value, whereas those shielded in the (S)-MTPA derivative will present a negative ∆δSR value.
|
\(\Delta \delta ^{SR} = \delta_s - \delta_R\) \(\Delta \delta ^{SR} > 0\), must be R2 \(\Delta \delta ^{SR} < 0\), must be R1 |
 |
Example: 1-phenylethanol
The two Mosher esters of an undetermined stereoisomer of 1-phenylethanol were prepared and the following data was gathered.

- If the methyl has a positive ∆δSR, where will it be on the structure shown below? Where will the Ph be?
- What is the stereochemistry of the original chiral center?
R OR S
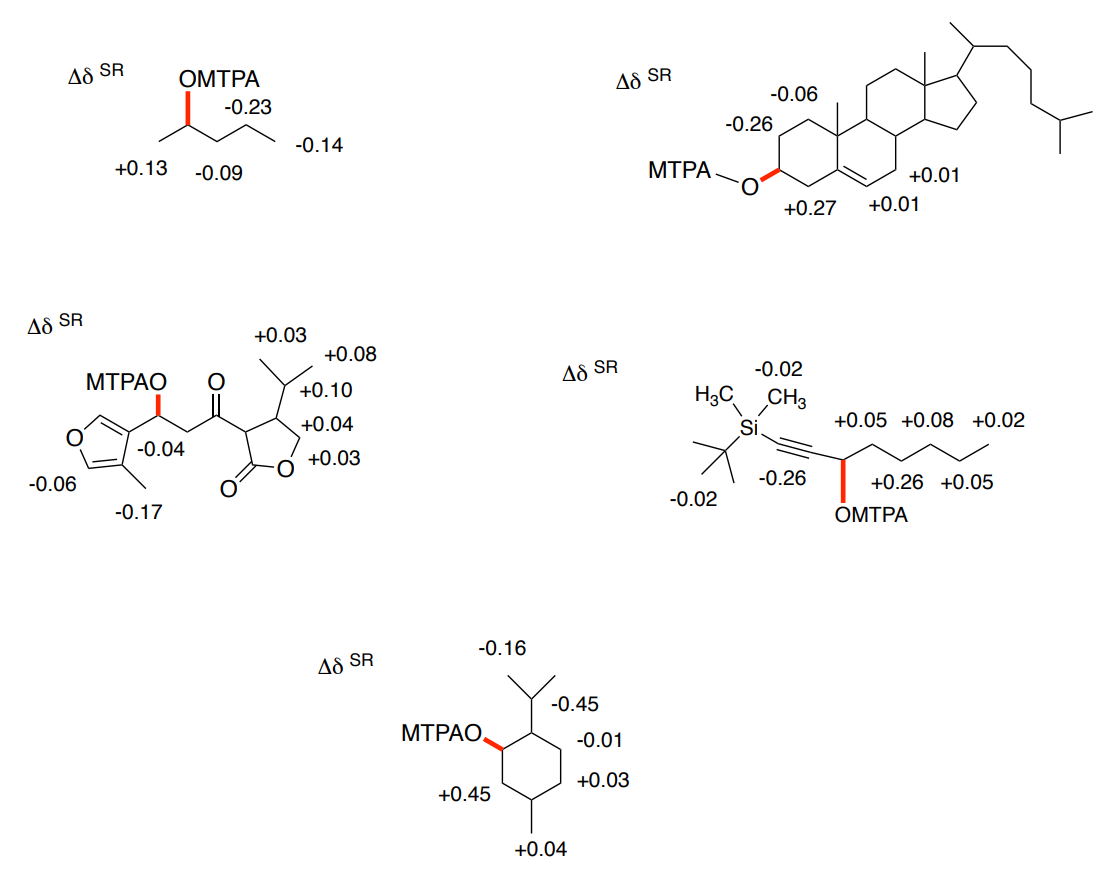
Practice Problems
- Using the following Mosher ester NMR data, calculate the stereochemistry of the starting alcohol.
Application Problem 1
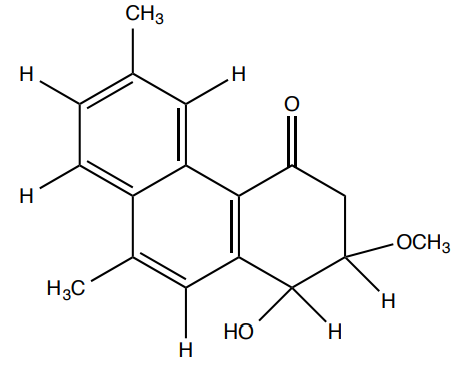
Heliotropium ovalifolium is an herbaceous plant responsible for several cases of cattle and sheep intoxications due to its content of alkaloids. Besides the known toxic products, researchers reported the presence of two antifungal and antibacterial benzoquinones from the aerial parts of the plant.
Answer: J. Nat. Prod., 2003, 66, 17-20.
- Circle all chiral centers in heliophenanthrone. How many possible stereoisomers are there?
- Use the 1H NMR data to determine the relative configuration.
- Coupling data: H-1 and H-2 (3JHH) 8.3 Hz
- No correlation was observed in the NOESY experiment between H-1 and H-2 signals.
- Use the following Mosher data to determine the absolute configuration.
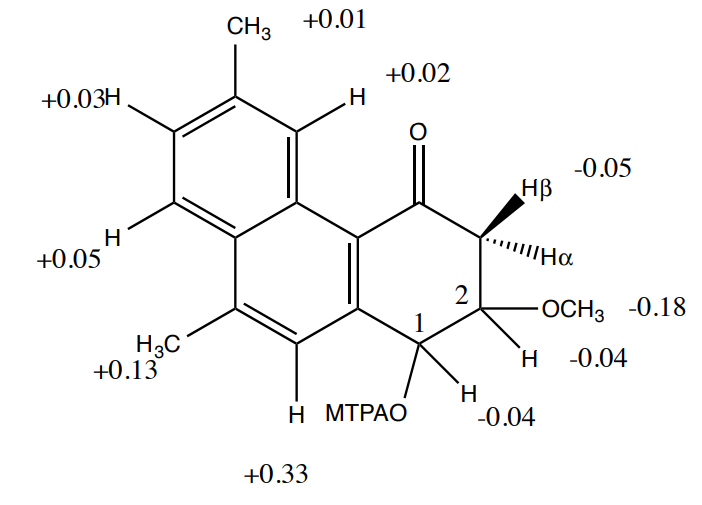
- Can you determine the chirality at all stereocenters using this data?


