The formal charge on an atom in a covalent species is the net charge the atom would bear if the electrons in all the bonds to the atom were equally shared. Alternatively the formal charge on an atom in a covalent species is the net charge the atom would bear if all bonds to the atom were nonpolar covalent bonds. To determine the formal charge on a given atom in a covalent species, use the following formula:
![]()
eg. 1: Carbon Atom in CH4
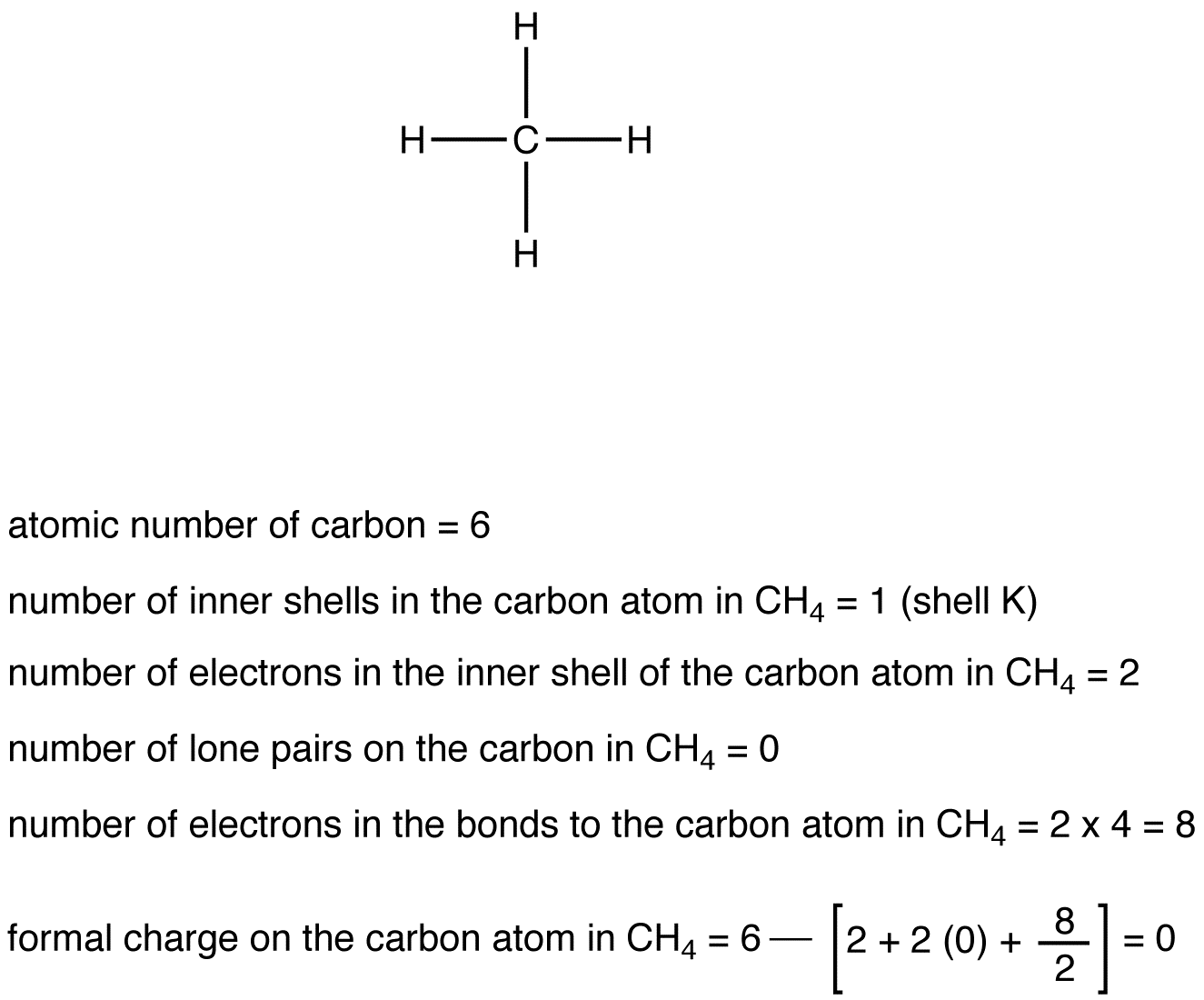
eg. 2: Carbon Atom in CH3
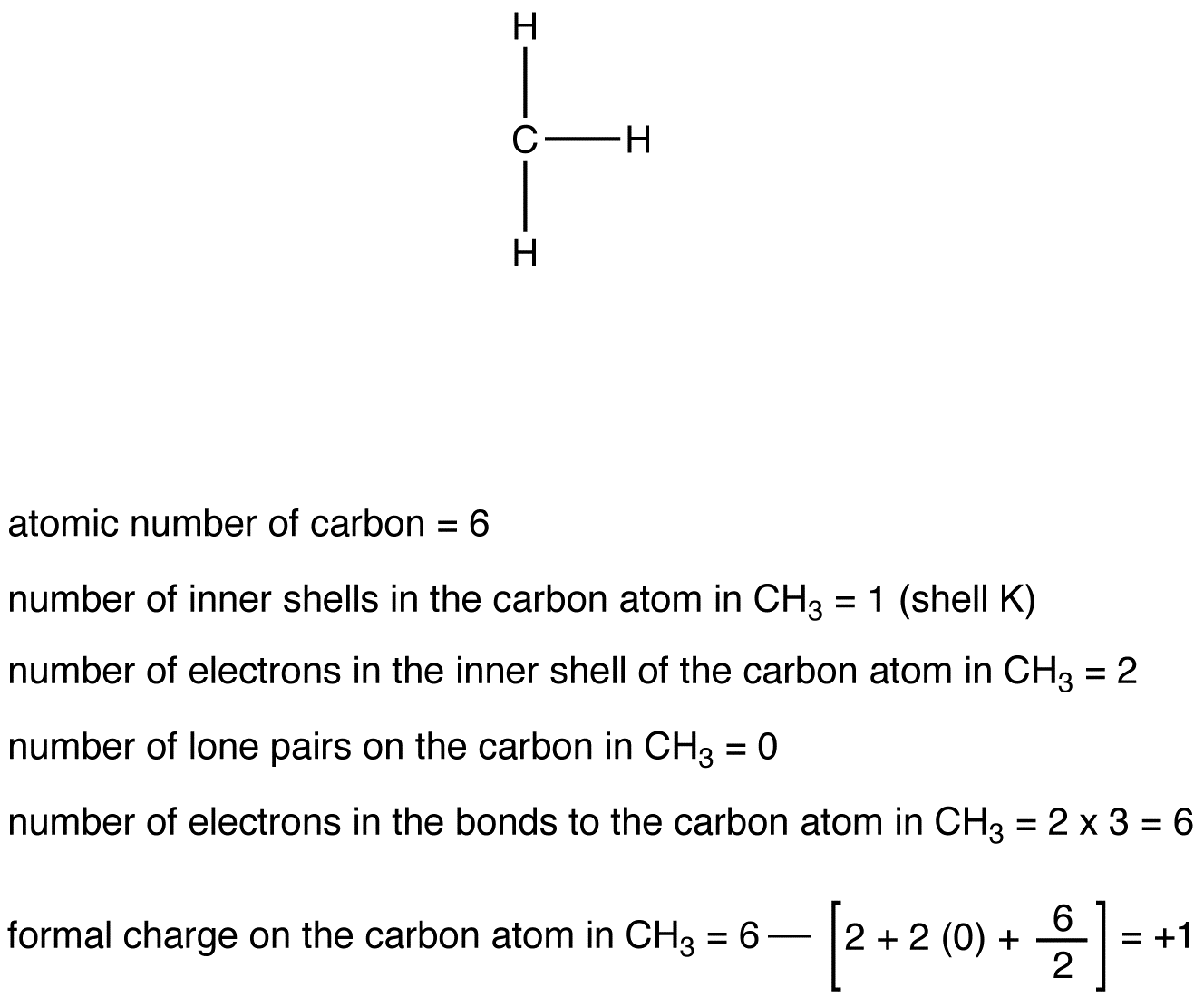
eg. 3: Carbon Atom in :CH3
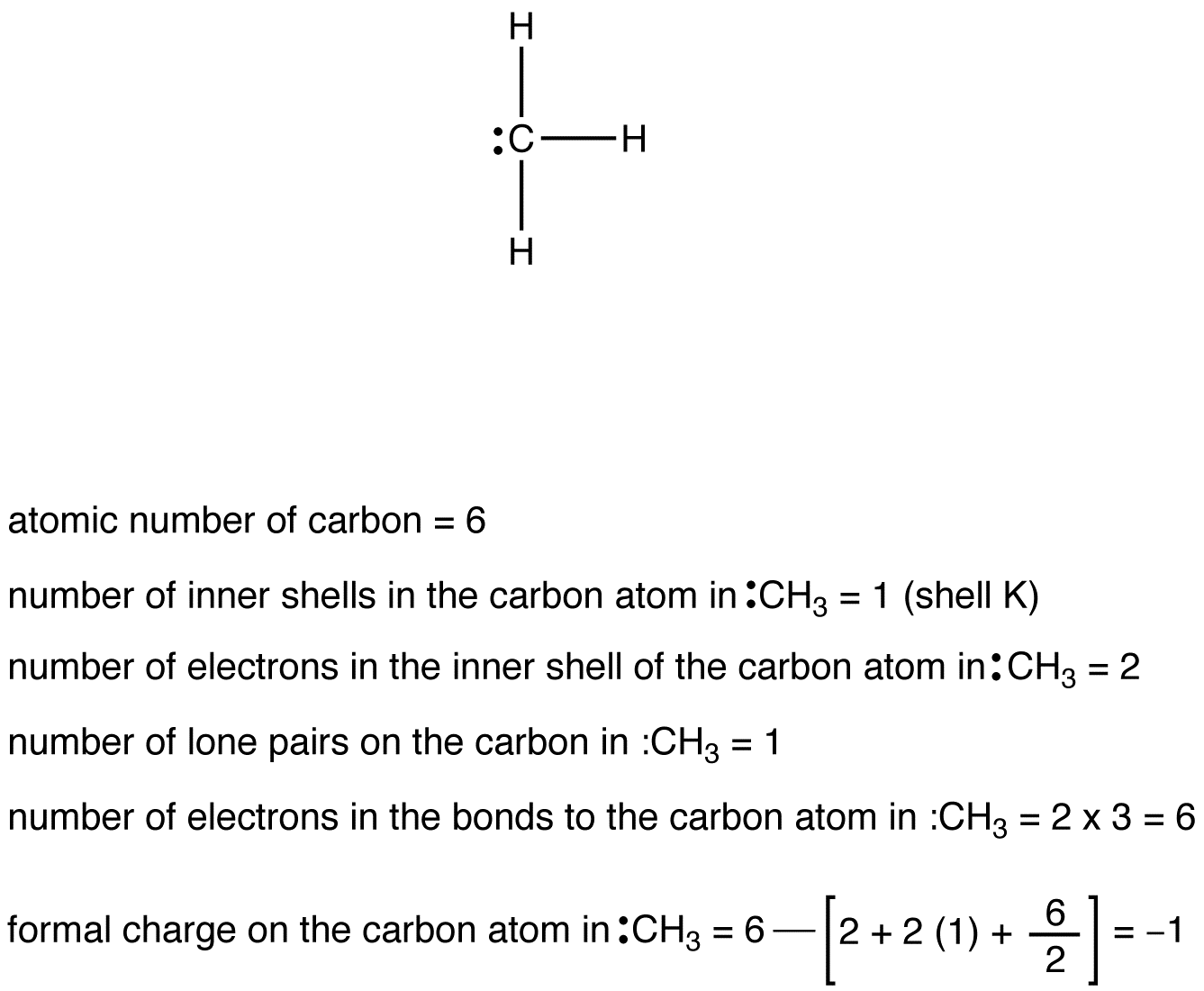
see also coordination number, oxidation number


