1.2: Reports
- Page ID
- 308523
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)First Report
Questions
- Tartaric acid and ester chemistry:
- What is the absolute configuration of natural L-tartaric acid in the Cahn-Ingold-Prelog nomenclature (R/S) system?
- Dimethyl (L)-tartrate can be purchased cheaply. Why would chemists choose to use the benzyl ester in this case? If methyl ester were used, in what part of the synthesis might side reactions be encountered, and what kinds of side reactions might be observed?
- The rate constant for the alkaline hydrolysis of the first methoxy group from dimethyl tartrate in water at 35 oC has been estimated to be 10 M-1 sec-1 [Genevois, L. Bull. Soc. Chim. Fr. 1937, 1506-1508.] Assuming this rate constant is accurate, if a 1M aqueous solution of dimethyl tartrate is kept a constant pH of 12 for 12 hours at 35 oC, what is the molarity of the remaining dimethyl tartrate?
- Diels-Alder chemistry:
- Outline a mechanism of the uncatalyzed Diels-Alder reaction between methacrolein and cyclopentadiene.
- Formulate from the product you have obtained from this reaction whether the relationship of the diene to the dienophile is exo or endo. Evaluate computationally at PM3 and ab-initio 3-21G* level of theory (see Appendix 1) the thermodynamic stability of exo-CHO-3 and endo-CHO-3 diastereoisomers. Attach the results to your first Report.
- To work with PC Spartan Pro 1.0.5. installed on the Dell Optiplex GX1 PC read the Appendix 1 (Ferrocene experiment) from the 5.311 manual. Run only optimization.21 Suggestions: Start calculation at semi-empirical PM3 level.

- When the computation has finished Save As... the file, for example as “BCH_CH3_exo_CHO_PM3” (BCH is from bicyclo[2.2.1]heptane, which is the carbon skeleton of the adduct). PM3 is a very quick computation, it takes ca 2 minutes. Fill in the Table below the heat of formation value (which are in kcal/mol).
- Then Save As…the same file, for example as “BCH_CH3_exo_CHO_321G”; start a new calculation at ab-initio level with 3-21G* basis set. It takes about 45 minutes to complete this calculation.
- Repeat the above steps (semi-empirical PM3, ab-initio 3-21G* and DFT/SVWN/DN* for BCH_CH3_endo_CHO_xxx”
- Fill in the Table below. Which diastereoisomer is more stable?
-
Diels-Alder adduct PM3 (kcal/mol) Ab-initio 3-21G* (a.u., hartree) DFT/SVWN/DN* (a.u., hartree) exo-CHO-3 endo-CHO-3 Energy difference: Eexo -Eendo (kcal/mol) 1 a.u.=623.5095 kcal/mol 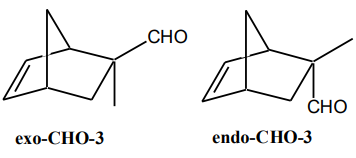
- Outline a probable mechanism for the Diels-Alder reaction in the presence of the asymmetric boronic ester/tartrate catalyst. What kind of interactions may be significant? The dimethoxyphenyl group plays a role in the transition state interactions, and such groups as tert-butyl in its place yield little if any enantioselection. Account for this fact in your postulated transition state structure. Calculate at PM3 level of theory the conformation of the complex chiral boron catalyst*methacrolein. Build the catalyst in two assumptions by starting from (i) L-tartaric acid and (ii) from D-tartaric acid. Establish in each case which face, re or si, of the CC double of methacrolein is available for cyclopentadiene addition. Attach the molecular pictures of the computed structure for case (i) and (ii) to your First Report.
- Does the favored absolute configuration of the product depend on whether the reaction proceeds an exo or an endo transition state? Explain. Does your mechanistic model suggest that an exo or an endo transition state prevails in the reaction of a-methylacrolein?
Final Report
You will be expected to provide a final (written or oral) report describing your experiments and observations. If your experiments deviate from those outlined in any significant way, a brief written outline of the experimental conditions and the results should be provided, since we are attempting to develop a new experiment for future use.
Footnotes
- For more information consult “PC Spartan plus. Tutorial and User’s Guide”

