1.1: Asymmetric Catalysis of Diels-Alder Reactions
- Page ID
- 308522
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Purpose
This experiment involves the preparation of mono(2,6-dimethoxybenzoyl)tartaric acid and its use as boronic acid catalyst (BLn*) for a Diels-Alder reaction.1 The preparation of the catalyst requires protection of the carboxyl groups of L-(+)- or D(-)-tartaric acid by esterification (reaction A),
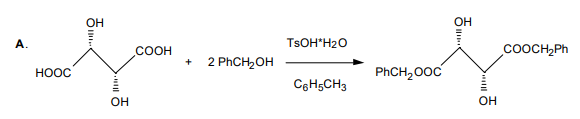
acylation of one of the two hydroxyl groups by 2,6-dimethoxybenzoyl chloride (reaction B.a.), and removal of the ester protection from the carboxyl groups (reaction B.b.).
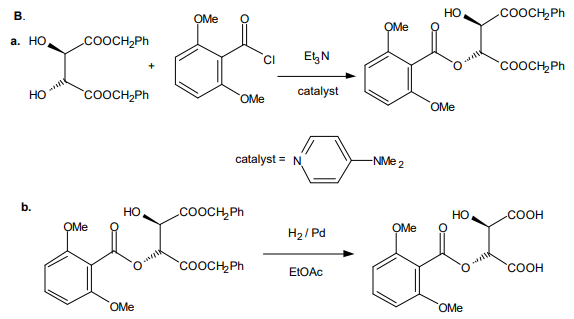
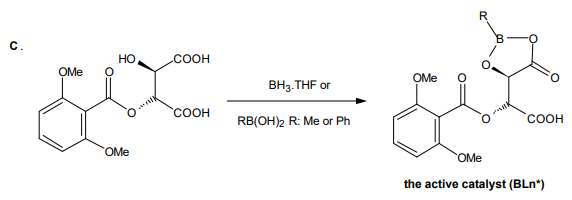
The mono(2,6-dimethoxybenzoyl)tartaric acid is purified and combined with borane or a boronic acid to form the active catalyst (reaction C, BLn*).
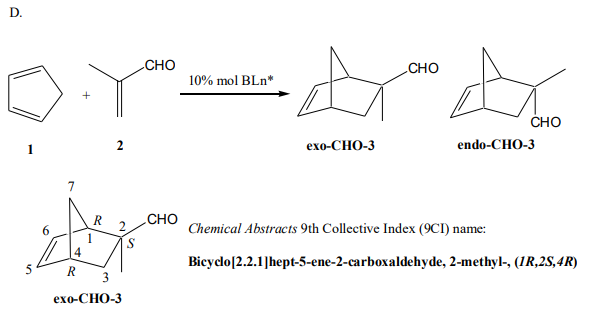
The Diels-Alder reaction of cyclopentadiene (1) with methacrolein (2), or of other diene/dienophile combinations, is carried out (reaction D). The product exo-CHO-3 is purified and its enantiomeric purity is measured.
References
Chemistry
See references in footnotes #1 and #2.
Technique
The following two references are strongly recommended to reside on your desk:
- Zubrick, J. W.; The Organic Chemist Lab Survival Manual: A Student Guide to Techniques; Wiley: New York, 1992.
- Leonard, J.; Lygo, B.; Procter, G.; Advanced Practical Organic Chemistry, 2nd ed.; Blackie Academic & Profesional: London, 1995.
Asymmetric Reactions2
General comments: Biological systems are made of asymmetric molecules, and therefore are affected differently by opposite enantiomers. There is a considerable interest in making single pure enantiomers for such purposes as pharmaceuticals as well as flavors and fragrances, insect pheromones, and other compounds of biological relevance. Racemic mixtures can often be resolved into separate enantiomers, but it is potentially more efficient to synthesize a single enantiomer.
Asymmetric synthesis requires a means of distinguishing one enantiomer from the other at the time the asymmetry is introduced. This distinction can only be made by molecules that are themselves asymmetric. A number of natural asymmetric compounds have been used as chirality sources, including alkaloids, sugars, amino acids, and terpenoids, but one most inexpensive and easily available is tartaric acid. Natural L-(+) tartaric acid will be used as the source of chirality in this experiment. “Unnatural” D-(-) tartaric acid is available via synthesis from a carbohydrate, though at several times higher cost, and as a consequence any synthesis based on tartaric acid as the source of asymmetry can be used to make either enantiomer of a product.
There are several ways in which an asymmetric source molecule can be used. The methods of organic synthesis allow conversion of natural materials to other molecules that retain the stereocenters of the original natural product. In such instances, the asymmetry can only be used once, and if multistep synthesis is required, the yield of the desired asymmetric product may be only a small fraction of the amount of the starting material required. More efficient utilization of the chirality source can be made if it is used as a chiral auxiliary, that is, if it is connected to the rest of the molecule at a critical stage of the synthesis to induce asymmetry. Once the asymmetry of an intermediate has been established, the chiral auxiliary can be disconnected (for example, by ester hydrolysis, or whatever means is appropriate for the system) and recycled.
The most efficient possible use of the asymmetric source molecule is to incorporate it into a catalyst. Catalysts by definition are not consumed in the reaction, and are recoverable after the desired reaction is over. As a practical matter, catalysts often degrade slowly as a result of side reactions, and recovery is not 100%, but one molecule of an efficient asymmetric catalyst may generate thousands of molecules of asymmetric product during its lifetime.
Tartrate3 chemistry: The catalyst to be used is derived either from L-tartaric acid or D-tartaric acid. The carboxyl groups are first protected by esterification with benzyl alcohol, one hydroxyl group is acylated with 2,6-dimethoxybenzoyl chloride, and benzyl ester groups are cleaved by hydrogenolysis. Benzyl esters are often used in place of methyl or ethyl esters when there is some reason that ester hydrolysis may not or is known not to yield the free acid without some kind of major side reaction. In general, hydrogen and palladium cleave benzyloxy groups to toluene and hydroxyl, often faster than double bonds are hydrogenated.
The acylated tartaric acid reacts with borane-THF to form a cyclic boronic acid derivative of the tartrate. Alternatively, phenyl boronic acid or methylboronic acid may be used to form tartrate derivatives. These cyclic boronic ester/anhydrides are active Lewis acid catalysts for promotion of Diels-Alder reactions and other kinds of reactions subject to acid catalysis, and the asymmetry of the tartrate induces a high degree of asymmetry in the products in favorable cases.
Diels-Alder Reactions (a more advanced discussion can be found in Appendix): These simultaneously make two carbon-carbon bonds, and if the product lacks a plane of symmetry, create four stereocenters in the process. Thus, a synthesis of a molecule containing several stereocenters via a Diels-Alder reaction may be particularly efficient, provided that the relative and absolute stereochemistry of the Diels-Alder reaction can be controlled. There is no general way of accomplishing this objective for all types of Diels-Alder reactions, but the catalyst developed recently in Hisashi Yamamoto’s group (Nagoya University) provides good stereocontrol with a number of useful combinations of substrates.
A classical example of a Diels-Alder reaction is that of maleic anhydride with cyclopentadiene. Because all the possible products contain a plane of symmetry, this is not a candidate for asymmetric control, but two kinds of relative stereoisomers result. The major product in this case is the endo isomer, and the minor product is the exo isomer. The endo transition state provides more extensive p-overlap than the exo, and accordingly stronger bonding between the two fragments. However, there are also many Diels-Alder reactions in which the exo isomer is favored for steric reasons.
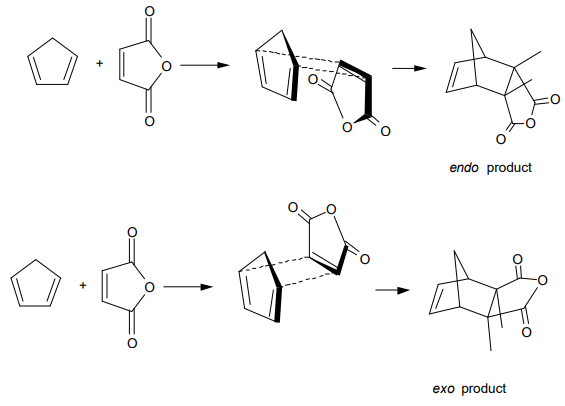
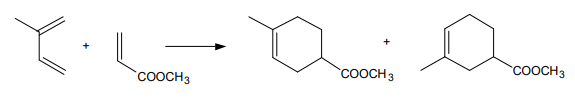
Diels-Alder reactions with asymmetrically placed substituents on each reactant provide possibility for regioisomerism.
Procedure4
Preparation of dibenzyl tartrate
L-Tartaric acid, D-Tartaric acid: Irritant!
Benzyl Alcohol: Severe Irritant! Harmful liquid! Target organ: nerves!
p-Toluenesulfonic acid monohydrate: Toxic! Corrosive! Oxidizer! Hygroscopic!
Avoid contact and inhalation! Caution: Contains sulfuric acid-may cause cancer by inhalation.
Toluene: Flammable liquid! Toxic!
|
Reagent |
Amount |
F.W. |
m.p. (b.p.) oC |
d g/mL |
|---|---|---|---|---|
| L-(+)-Tartaric acid or D-(-)- Tartaric Acid | 3.0 g (20 mmol) | 150.09 | 171-174 (dec) | |
| Benzyl alcohol | 6.5 g (60 mmol) | 108.14 | -15 (205) | 1.045 |
| TsOH*H2O | 47.5 mg (0.25 mmol) | 190.22 | 103-106 | ------ |
| Toluene | 40 mL | 92.14 | -93 (110.6) | 0.865 |
In a 100 mL, one necked, round-bottomed flask equipped with a magnetic stirrer, a Hickman still and a reflux condenser are placed 3.0 g either L-(+)-tartaric acid (“natural” tartaric acid) or D-(-)-tartaric acid, benzyl alcohol (6.5 g), 40 mL toluene, and
Note
Make sure to record which isomer you’re using
47.5 mg p-toluenesulfonic acid monohydrate. The mixture is stirred and heated (heating mantel) under reflux overnight. Water (theory: 0.72 mL) is collected in the Hickman still (should be tilted slightly to collect water). The mixture is allowed to cool to room temperature, diluted with ether (50-100 mL), and poured into 50 mL of saturated aqueous sodium bicarbonate.
CAUTION: Gas evolution and possible foaming is expected
The phases are separated in a separatory funnel and the aqueous phase is extracted with two 20-mL portions of ether. The organic solutions are combined, dried over anhydrous sodium sulfate, filtered, and concentrated in a rotary evaporator. The residue is triturated5 with a mixture of 200 mL of hexane and 10 mL of ether to yield white crystalline (-)-dibenzyl tartrate. The crystalline product is collected by filtration and washed with a mixture of 20 parts hexane to 1 part ether. Concentration of the filtrate yields a second crop of a crystalline dibenzyl tartrate. The total yield is 6.2 g (94 %), m.p. 49-50 °C; the 1H-NMR spectrum should be recorded,6 \( [\alpha]^{24}_D \) -10.2o (CHCl3, c 1.03).
Preparation of dibenzyl mono(2,6-dimetoxybenzoyl)tartrate
Dibenzyl tartrate:
Dichloromethane : Toxic Irritant!
Triethylamine : Flammable liquid! Corrosive!
4-Dimethylaminopyridine7 : Highly toxic! Corrosive! Readily absorbed through skin! Avoid contact and inhalation!
2,6-Dimethoxybenzoyl chloride : Corrosive! Moisture-Sensitive!
Silica gel (70-230 mesh): Harmful dust! Avoid inhalation! Hygroscopic!
| Reagent | Amount | F.W. g/mol | m.p. (b.p.) oC | d g/mL |
|---|---|---|---|---|
| Dibenzyl tartrate | 6.1g (18.5 mmol) | 329.7 | 49-50 | |
| Dichloromethane | 100 mL | 84.93 | -97 (40) | 1.325 |
| Triethylamine | 4 mL, (28.8 mmol) | 101.19 | -115 (88.8) | 0.726 |
| 4-dimethylaminopyridine | 50 mg, (0.4 mmol) | 122.17 | 112-114 | |
| 2,6-Dimethoxybenzoyl chloride | 3.65 g, (18.2 mmol) | 200.62 | 64-66 |
A 250-mL three-necked round-bottom flask is equipped with a magnetic stirrer and a reflux condenser, with a nitrogen inlet capping the top of the condenser.8 In the flask are placed 6.1 g dibenzyl tartrate, 100 mL dichloromethane, 4 mL triethylamine, and 50 mg 4- dimetylaminopyridine. The mixture is stirred and cooled to 0 oC. Then 3.65 g9 of 2,6- dimethoxybenzoyl chloride is added10 in five approximately equal portions at 10-minutes intervals. The mixture is then warmed to reflux temperature and stirred under reflux overnight. The progress of the reaction may be monitored by TLC [mixture of hexane, ether, and dichloromethane (ratio 3:1:5) the Rf value is about 0.35]. After cooling to room temperature, the mixture is poured in 100 mL water and the phases are separated in a separatory funnel. The aqueous phase is extracted with two 75-mL portions of dichloromethane. The combined organic solutions are dried over the minimum required amount of sodium sulfate, filtered, and concentrated on a rotary evaporator to yield a viscous oil.11 This material purified by flash column chromatography (see Appendix 3) on silica gel (~100 mL), eluting with a mixture of hexane, ether, and dichloromethane

(ratio 3:1:5). Concentration yields 7.1-7.5 g, 78-82% oily liquid dibenzyl mono(2,6- 24 dimethoxybenzoyl) tartrate; the NMR spectrum should be recorded;12 \( [\alpha]^{24}_D \) -60.1o (CHCl3, c 1.15).
Preparation of mono(2,6-dimethoxybenzoyl)tartrate.
10% Palladium on charcoal: Flammable solid! Irritating dust! Store under nitrogen! Often ignites solvents such as ethyl acetate if added to the solvent in air!13
Ethyl acetate: Flammable liquid (Fp -3 oC)! Irritant!
| Reagent | Amount | F.W. | m.p. (b.p.) oC | density |
|---|---|---|---|---|
| 10 wt % Pd on activated charcoal | 580 mg | - | - | |
| Ethyl acetate | 100 mL | 88.11 | -84 (76.5-77.5) | 0.902 |
| dibenzyl mono(2,6- dimethoxybenzoyl)tartrate | 5.8 (11.7 mmol) | 495.73 |
A three-neck 250-mL round-bottomed flask equipped with septum caps, condenser (used as a spacer), magnetic bar, two hydrogen balloons connected to the branches of a Tstopcock (see Fig. 3, the T stopcock is open such as that only the two balloon communicate) mounted on the top of the condenser. The apparatus is first flushed with nitrogen. Nitrogen flows into a bubbler then into the round bottom flask via a syringe needle inserted in the side arm septum. A second syringe needle, used for venting, is inserted into the septum covering the second neck. The nitrogen flow rate is adjusted so that a bubble escapes through the bubbler every few seconds. Allow the nitrogen flushing to continue for 4-5 minutes. Under slight positive nitrogen pressure, remove the septum (with the inserted vent needle) and charge the flask with 580 mg 10% palladium on charcoal, 100 mL ethyl acetate (see footnote #24) and 5.8 g dibenzyl mono(2,6- dimethoxybenzoyl)tartrate. After the reagents are in the flask, cap the flask with the rubber septum (and the vent needle) and continue to flush with nitrogen 3-4 minutes more. Remove the nitrogen-in and the exit-needle. Turn the T stopcock such as that hydrogen can enter into the round bottom flask. Leave the hydrogenation overnight (come back the next day to refill balloons if necessary) or over the weekend. The progress of the hydrogenolysis is monitored by TLC. The mixture is filtered through a pad of Celite.14 SAVE the collected palladium catalyst in a special jar for palladium wastes for recycling.
Caution
Palladium catalyst sometimes ignite during filtration!
The filtrate is concentrated on a rotary evaporator. The residue is thoroughly dried15 under vacuum at 80 oC, < 1 torr (vacuum pump) over night, yielding practically pure solid mono(2,6-dimethoxybenzoyl)tartric acid, m.p. 184-186 oC, suitable for use in the next step; 1H-NMR should be recorded16; \( [\alpha]^{24}_D \) -73.0o (EtOH, c 1.03).
Preparation of (1R,2R,4R)- or (1R,2S,4R)-exo-2-Methylbicyclo[2.2.1]hept-5-ene2-carboxaldehyde
Dichloromethane : Toxic Irritant!
BH3-THF: Flammable liquid (Fp -17 oC)! Moisture-sensitive!
Metacrolein: Flammable liquid (Fp-15 oC)! Corrosive!
Cyclopentadiene (freshly distilled by the TAs): Flammable liquid!
| Reagent | Amount | F.W. | m.p. (b.p.) oC | density |
|---|---|---|---|---|
| Mono(2,6- dimethoxybenzoyl) tartaric acid | 1.57 g (5 mmol) | 314.0 | ||
| Dichloromethane | 50 mL | 84.93 | -97 (40) | 1.325 |
| BH3 -THF | 5 mL, 1M (5 mmol) | 85.94 | 0.898 | |
| methacrolein | 4.14 mL (50 mmol) | 70.09 | -81 (69) | 0.847 |
| cyclopentadiene | 5 mL (60 mmol) | 66.10 | -85 (41.5-42) | 0.8021 (@ 20 °C) |

A 125-mL three-necked tapered flask is equipped with a stirring bar, Vigreux column that carries on the top through a Teflon adapter a 3-way stopcock, low temperature thermometer in the threaded side neck, and a rubber septum covering the second side neck (see Fig. 4). The stopcock side arms are connected to nitrogen and to a bubbler. The air is replaced by repeated flushing with nitrogen, first (3-5 minutes) using a vent syringe needle inserted in the septum followed by continuing the nitrogen flushing for an additional 4 minutes after the vent needle has been removed. Remove the septum and under a slight positive nitrogen pressure the flask is charged with 1.57 g mono(2,6- dimethoxybenzoyl)tartaric acid and 50 mL dichloromethane (previously stored on 4 A molecular sieves). Put the septum back securely. The mixture is cooled with an ice bath. Turn the T stopcock such as to shut off the incoming nitrogen. Borane-THF solution (5 mL of 1M, 5 mmol) is added dropwise from a syringe (with the needle through the rubber septum) to a stirred, cooled solution over a period of 30 min.
Caution
Borane-THF is highly flammable and evolves hydrogen on contact with water and hydroxylic solvents.
Vigorous hydrogen evolution occurs during the addition can be noticed through the bubbler. Stirring at 0 oC is continued for 15 min. after the addition is complete. Reconnect the nitrogen-in flow by turning the T-stopcock into the appropriate position. The mixture is then cooled with a dry ice acetone bath (-78 oC). Freshly distilled methacrolein (4.14 mL) is added dropwise from a syringe,
Caution
Methacrolein is toxic and volatile and should be handled strictly in the hood.
followed by 5 mL cyclopentadiene. The mixture is stirred well and kept at -78 oC for 12 hours. (A large Dewar half-filled with dry ice will allow 24-hour storage.) The cold mixture is poured into 150 mL of ice-cooled saturated sodium bicarbonate solution). The product is extracted with three 200-mL portions of hexane. The sodium bicarbonate solution is saved for catalyst recovery, and the combined hexane solutions are washed with brine (i.e., saturated sodium chloride), two 200-mL portions, dried over MgSO4 and concentrated on a rotary evaporator. The crude adduct (yield 85%) consists of an endo/exo mixture ratio of 11/89 (verify this information by taking a 1 H NMR of the crude adduct in CDCl3).17 The endo-CHO-3 adduct was removed by column chromatography (24:1 hexane/ethyl acetate) to give exo adduct 3 as a colorless solid (bp 74-75 oC /15 Torr; mp 67-68 oC18). The Diels-Alder adduct has R configuration if the catalyst derived from natural tartaric acid; the S isomer results from a catalyst derived from unnatural tartaric acid. The NMR spectrum of pure exo-CHO-3 should be recorded19; \( [\alpha]^{20}_D \) -23.3o (ethanol).20
Catalyst recovery: The sodium carbonate solution containing the mono(2,6- dimethoxybenzoyl)tartaric acid is acidified with 4 M HCl (gas evolution) and extracted with three portions (~ 50 mL) ethyl acetate. Concentration of ethyl acetate solution and vacuum drying yields the recovered catalyst (~1.57 g, 97%), which needs no further purification.
Other Diels-Alder Reactions
As time permits, other Diels-Alder reactions can be tested with any of the above described catalyst systems. Methyl acrylate, methyl methacrylate, acrylic acid, methacrolein, or other similar compounds might be tested with 2,3-dimethylbutadiene, isoprene, cyclopentadiene, cyclohexadiene, or other available dienes.
Measurement of enantiomeric excess
Although optical rotation gives a rough measurement of enantiomeric excess if the rotation of the pure enantiomer is known, rotation measurements are subject to considerable experimental error. Rotations in the literature are often inaccurate. Before NMR and chromatography were commonly available, literature rotations were generally the highest rotation anyone had obtained on the purest sample that had been made. Resolutions were considered complete when rotation no longer increased on recrystallization of the combination of the resolving agent and compound, but false plateaus have frequently occurred. Impurities usually lower rotations, but sometimes a diastereoisomer or optically active impurity with a high rotation can increase the observed rotation. The experimental sample also has to be highly purified, with the same errors possible.
More accurate measurements of enantiomeric purity can be made with the aid of a chiral auxiliary and NMR or gas chromatographic analysis. For example, the enantiomeric purity of (1R,2R,5R)-exo-2-methylbicyclo[2.2.1]hept-5-ene-2-carboxaldehyde can be measured by making a cyclic acetal with (2R,4R)-2,4-pentanediol as described by Furuta, Gao and Yamamoto (see ref. in footnote #1). Similar derivatives of most aldehyde can be made. Acids are converted to esters of chiral alcohols. Alternatively, an NMR spectrum can be taken in the presence of chiral shift reagent or/and GC on chiral column (see Appendix 2). It will be of interest to measure the enantiomeric excess of any synthesized sample of Diels-Alder adduct if time permits.
Footnotes
- Furuta, K.; Shimizy, S.; Miwa, Y.; Yamamoto, H. J. Org. Chem. 1989, 54, 1481.
- For futher reading see: (a) Procter, G. Asymmetric Synthesis, Oxford University Press: Oxford, 1996: Chapts. 1,2 and 6 (QD262.P76); (b) Atkinson, R. S. Stereoselective Synthesis, Wiley: New York, 1995, Chaps.1 and 6 (QD481.A79); (c) Eliel, E. L. Wilen, S. H. Stereochemistry of Organic Compounds, Wiley, New York, 1994. (QD.481.E).
- Gawronski, N.; Gawronska, K. Tartaric and Malic Acid in Synthesis, Wiley: New York, 1999 (QD305.A2.G217).
- Adapted after: Furuta, K.; Gao, O.-z.; Yamamoto, H. Org. Synth. 1995, 72, 86-94.
- To rub, crush, grind, or pound into fine particles or a powder; pulverize. A triturated substance, especially a powdered drug.
- From Org. Synth: 1 H-NMR (CDCl3, 200 MHz, d): 3.15 (d, 2H, J=7.5), 4.6 (d, 2H, J=7.5), 5.27 (s, 4H), 7.35 (s, 10H).
- Widely used and versatile hypernucleophilic acylation catalyst.
- Use of a three-necked flask allows easier access when material is added or withdrawn for TLC, but except during these operations, two of the necks are merely stoppered (see Fig. 2). The nitrogen inlet should be connected to a T-stopcock, with a second branch of the tube connected to the system and the third branch be connected to a bubbler. The nitrogen flow rate is adjusted so that a bubble escapes through the bubbler every few seconds. Use at the beginning of the flushing a vent syringe needle inserted in the septum. Then, remove the needle and allow the nitrogen flushing to continue another 2-3 minutes.
- Because the acid chloride supplied by Aldrich is only 90% pure, this calculation as published in Org. Synth. is incorrect. It is possible that the yield can be increased by 10% and the purification of the product made easier if the amount of acid chloride is increased to a true 18.2-18.5 mmol. The effect of small excess, up to 20 mmol total, would also be of interest. The student record the amount actually used.
- If a three-neck flask is used, one stopper may be removed briefly while material is added. If a onenecked flask is used, the nitrogen connection is separated momentarily and the acid chloride is added through the top of the reflux condenser.
- Although the Org. Synth. prep does not call for it, it is suggested that the oil be dissolved in diethyl ether and extracted with three portions of saturated sodium bicarbonate to remove carboxylic acid impurities before chromatography. It is of interest to check the purity of the material by 1 H-NMR at each stage
- From Org. Synth: 1 H-NMR (CDCl3, 200 MHz, d): 3.24 (d, 1H, J=9); 3.7 (s, 6H); 4.89 (dd, 1H, J=2.5 and 9); 5.08-5.34 (4H); 5.99 (d, 1H, J=2.5); 6.53 (d, 2H, J=8.5), 7.25-7.4 (m, 11H).
- A good procedure is to put the catalyst in the empty flask, flush the flask with a gentle stream of nitrogen, and cover the catalyst with a part of solvent. The remainder of the solvent may be used to aid transfer of the tartrate ester. As a safety measure, before introducing hydrogen into the flask, be sure that all of the catalyst in under the solution, and flush the apparatus with nitrogen before introducing hydrogen.
- Finely divided catalyst tends to plug filters.
- Thorough drying is essential to obtain the % ee as described.
- From Org. Synth: 1 H-NMR (CDCl3, 200 MHz, d): 3.63 (s, 6H); 4.61 (d, 1H, J=2); 5.61 (d, 1H, J=2); 6.38 (d, 2H, J=9), 7.13 (t, 1H, J=9).
- 1 H NMR (300 MHz, CDCl3 d): 9.33 (s, 1H, CHO, endo); 9.56 (s1H, CHO, exo); see: Ishihara, K.; Kurihara, H.; Matsumoto, M.; Yamamoto, H. J. Am. Chem. Soc. 1998, 120, 6920-6930.
- Beckmann, S.; Bamberger, R. Liebigs Ann. Chem. 1953, 580, 198.
- From: Ishihara, K.; Kurihara, H.; Matsumoto, M.; Yamamoto, H. J. Am. Chem. Soc. 1998, 120, 6920- 6930: exo-2-Methylbicyclo[2.2.1]hept-5-ene-2-carboxaldehyde: 1 H-NMR (CDCl3, 300 MHz, d): 0.76 (d, J=12.0 Hz, 1H); 1.01 (s, 3H), 1.38-1.40 (m, 2H); 2.25 (dd, j=3.8, 5.7 Hz, 1H), 2.82 (bs, 1H); 2.90 (bs, 1H), 6.11 (dd, j=3.0, 5.7 Hz, 1H), 6.30 (dd, 1H, J=3.0, 5.7 Hz, 1H); 9.69 (s, 1H).
- Hashimoto, S.; Komeshima, N.; Koga, K. J. Chem. Soc., Chem. Commun., 1979, 437.

