6: Qualitative Analysis of Group I Ions (Experiment)
- Page ID
- 96002
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To follow a classic analytical scheme to separate and identify the ions in a known mixture of Group I cations
- To then apply this scheme to identify the ions in an unknown mixture of Group I cations.
One common task in analytical chemistry is the identification of the various ions present in a particular sample. For example, if you are an environmental chemist your job may be to recover soil or water samples in order to determine the presence of toxic ions such as \(\ce{Pb^{2+}}\) or \(\ce{Hg2^{2+}}\). A common experimental method used to identify ions in a mixture is called qualitative analysis.
In qualitative analysis, the ions in a mixture are separated by selective precipitation. Selective precipitation involves the addition of a carefully selected reagent to an aqueous mixture of ions, resulting in the precipitation of one or more of the ions, while leaving the rest in solution. Once each ion is isolated, its identity can be confirmed by using a chemical reaction specific to that ion.
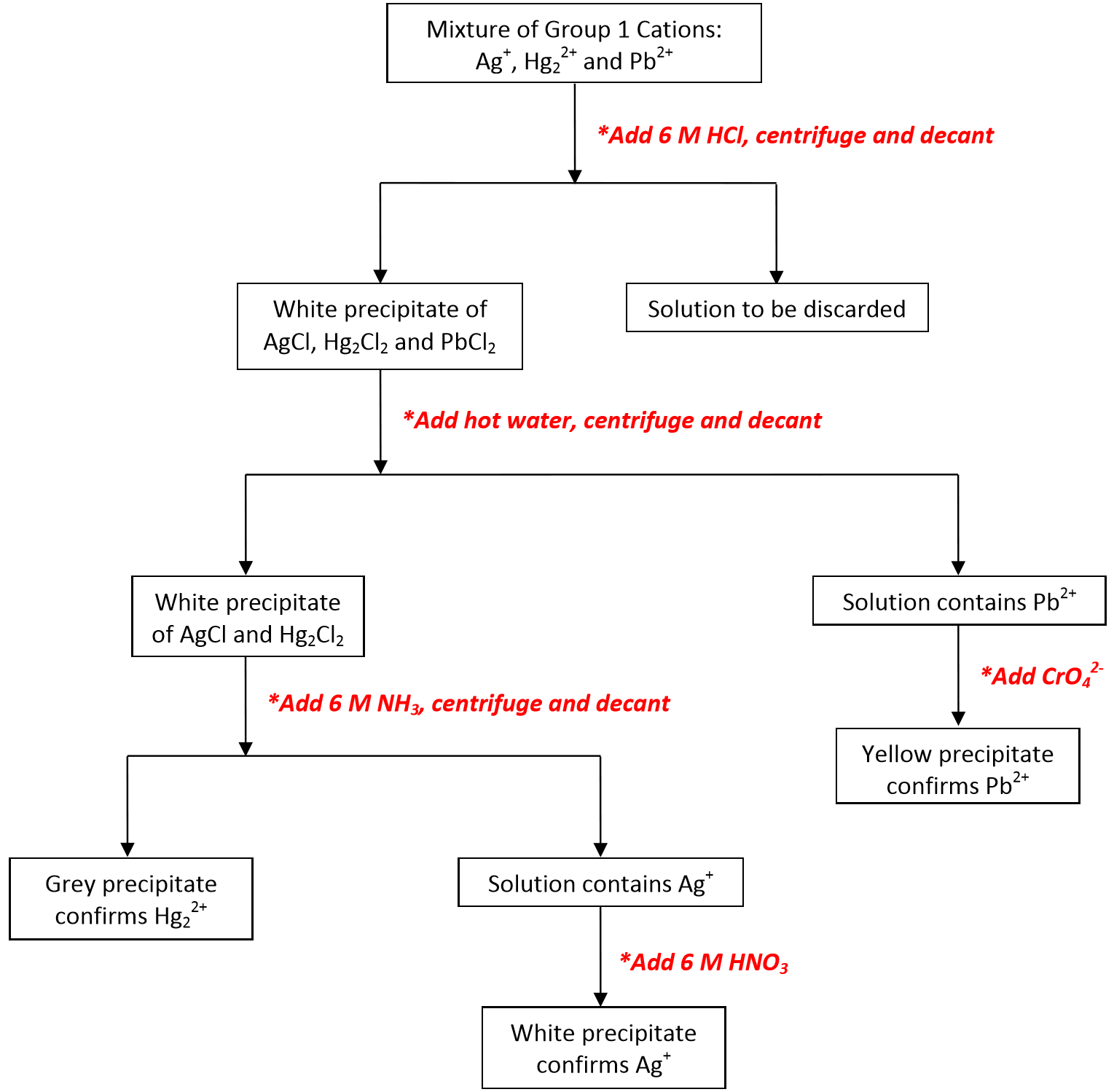
Cations are typically divided into Groups, where each group shares a common reagent that can be used for selective precipitation. The classic qualitative analysis scheme used to separate various groups of cations is shown in the flow chart below.
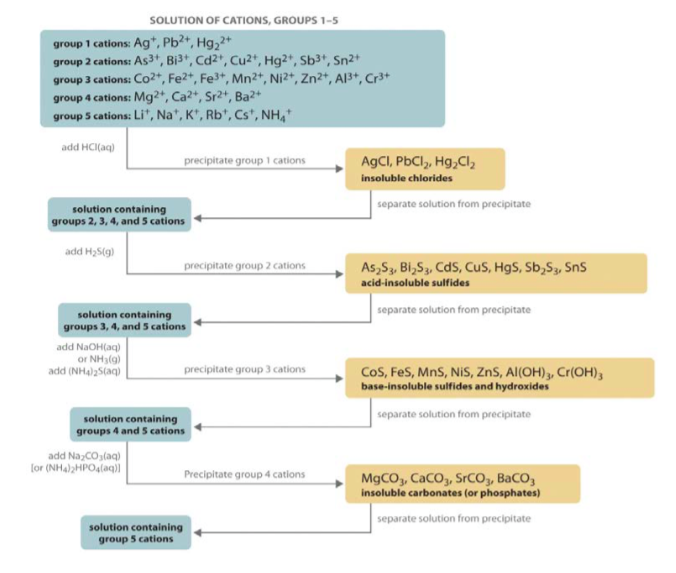
Note that \(\ce{Ag^{+}}\), \(\ce{Pb^{2+}}\), and \(\ce{Hg2^{2+}}\) are called the Group I cations since they are the first group separated from the larger mixture. Since these ions all form insoluble chlorides, their separation from all other ions may be accomplished by the addition of 6 M \(\ce{HCl}\) (aq) resulting in the precipitation of \(\ce{AgCl}\) (s), \(\ce{PbCl2}\) (s), and \(\ce{Hg2Cl2}\) (s):
\[\ce{Ag^{+} (aq) + Cl^{-} (aq) -> AgCl (s)} \label{1}\]
\[\ce{Hg2^{2+} (aq) + 2 Cl^{-} (aq) -> Hg2Cl2 (s)} \label{2}\]
\[\ce{Pb^{2+} (aq) + 2 Cl^{-} (aq) -> PbCl2 (s)} \label{3}\]
The sample must then be centrifuged (spun rapidly), which separates the solid precipitates from the ions still in solution. The solids settle to the bottom, and the solution containing the remaining ions (Groups 2 – 5) remains on top of the solid. This solution is called the supernatant solution, and must be carefully decanted (poured off without disturbing the solid), and saved for further study. The Group I cations contained within the collected precipitate must then be separated from each other in order for the presence of each ion to be confirmed.
Separation and Confirmation of Group I Cations
Lead(II) chloride can be separated from the other two chlorides based on its increased solubility at higher temperatures. This means that lead(II) chloride will dissolve in hot water, leaving the mercury(I) chloride and the silver chloride in solid form:
\[\ce{PbCl2 (s) -> Pb^{2+} (aq) + 2 Cl^{-} (aq)} \label{4}\]
The presence of \(\ce{Pb^{2+}}\) in the aqueous solution can then be confirmed by the formation of a yellow precipitate of \(\ce{PbCrO4}\) upon the addition of aqueous \(\ce{K2CrO4}\):
\[\ce{Pb^{2+} (aq) + CrO42^{-} (aq) -> PbCrO4 (s)} \label{5}\]
Next, \(\ce{Hg2^{2+}}\) and \(\ce{Ag^{+}}\) cations can be separated by adding 6 M \(\ce{NH3}\) (aq) to the solid mixture of the two chlorides. Silver chloride will dissolve since it forms a soluble complex ion with ammonia:
\[\ce{AgCl (s) + 2 NH3 (aq) -> Ag(NH3)2+ (aq) + Cl^{-} (aq)} \label{6}\]
However, mercury(I) chloride reacts with the ammonia yielding what appears to be a gray solid which is actually a mixture of black \(\ce{Hg}\) (l) and white \(\ce{HgNH2Cl}\) (s). The presence of this gray solid is confirmation of the presence of \(\ce{Hg2^{2+}}\).
\[\ce{Hg2Cl2 (s) + 2 NH3 (aq) -> Hg (l) + HgNH2Cl (s) + NH4^{+} (aq) + Cl^{-} (aq)} \label{7}\]
The presence of \(\ce{Ag^{+}}\) can be confirmed by the appearance of a white precipitate upon adding 6 M \(\ce{HNO3}\) (aq) to the solution. The nitric acid reacts with the ammonia and thus destroys the complex ion containing the silver cation. Once in solution again the silver cation precipitates with the chloride as indicated by the following reactions:
\[\ce{Ag(NH3)^{2+} (aq) + 2 H^{+} (aq) -> Ag^{+} (aq) + 2 NH4^{+} (aq)} \label{8}\]
\[\ce{Ag^{+} (aq) + Cl^{-} (aq) -> AgCl (s)} \label{9}\]
The analysis scheme is represented in abbreviated form using the flow chart below:
Procedure
Chemicals
0.1 M \(\ce{AgNO3}\) (aq), 0.2 M \(\ce{Pb(NO3)2}\) (aq), 0.1 M \(\ce{Hg2(NO3)2}\) (aq), 6 M \(\ce{HCl}\) (aq), 6 M \(\ce{HC2H3O2}\) (aq), 1 M \(\ce{K2CrO4}\) (aq), 6 M \(\ce{NH3}\) (aq), 6 M \(\ce{HNO3}\) (aq), unknown sample
Equipment
8 small test tubes, glass stirring rod, small 10-mL graduated cylinder, 250-mL beaker, stand, ring clamp, wire gauze, small watch glass, dropper pipets, blue litmus paper, wash bottle filled with deionized water, Bunsen burner, centrifuge
- The PPE for this lab includes safety goggles, lab coat and nitrile gloves.
- Exercise appropriate caution when using all acids (hydrochloric acid, nitric acid and acetic acid) as they can cause serious chemical burns to your skin. If any acid comes into contact with your skin or eyes, immediately rinse the affected areas with copious amounts of water for 15 minutes, and inform your instructor.
- Also use caution when using potassium chromate and base solutions. If any comes into contact with your skin or eyes, immediately rinse the affected areas with copious amounts of water for 15 minutes, and inform your instructor.
Waste Disposal
All the Group I cations and the chromate anion are hazardous to the environment, thus all waste solutions containing these ions must be disposed of in the hazardous-waste container in the fume hood. Rinse all glassware directly into the waste container using a small squirt bottle to be certain all hazardous waste ends up in the waste container.
General Instructions
In this lab you will first prepare a solution containing all three Group I cations and analyze it by following the above procedure. The results you obtain will serve as a “positive control”. Then you will be supplied with an unknown sample which contains one, two, or perhaps even all three of the Group I cations. You will determine which cations are present in the sample by following this procedure a second time, and by comparing the results you obtain to those observed (positive tests) in the known control mixture.
To get correct results in this lab, good organizational skills and techniques are essential. Be sure to label all tubes and solutions because these accumulate rapidly, and it is very easy to get tubes and/or solutions mixed up. It is also suggested that students keep a “waste” beaker near their work area in which to discard all solutions locally, and then pour their combined waste solutions into the large waste container provided by the instructor at the end of lab.
Finally, be sure to clean out and thoroughly rinse all glassware (including stirring rods!) with deionized water between uses. Cross-contamination is one of the most common causes for false observations leading to incorrect conclusions.
Part A: Analysis of Known Mixture of Group I Cations – A Positive Control Experiment
Precipitation of Group I Cations
- Prepare a mixture of Group I cations by adding 1.0 mL of each of the following aqueous solutions to a small test tube: 0.1 M \(\ce{AgNO3}\), 0.2 M \(\ce{Pb(NO3)2}\) and 0.1 M \(\ce{Hg2(NO3)2}\). Note that 1.0 mL is generally between 10-15 drops.
- Pour 1.0 mL of the above mixture into a second small test tube and then add 2 drops of 6 M \(\ce{HCl}\) to this test tube.
- Centrifuge the mixture from Step 2 in order to completely separate the solid precipitate from the solution. Make sure that you balance the centrifuge before you start. Balancing is done by placing a test tube containing water opposite to the test tube containing the precipitate in the centrifuge. The two test tubes should have approximately equal weights.
- Add one more drop of \(\ce{HCl}\) to ensure that the precipitation is complete. If more precipitate is observed then centrifuge the mixture again.
- Decant the supernatant solution into another small test tube and save the precipitate for further study. The supernatant solution may be discarded in the lab waste container.
Separation of \(\ce{Pb^{2+}}\) from \(\ce{Hg2^{2+}}\) and \(\ce{Ag^{+}}\)
- Wash the precipitate from Step 5 to remove contaminates that may be trapped within the solid. This can be accomplished by adding 1.0 mL of deionized water, stirring, and then centrifuging. Once again decant the supernatant solution into a small test tube. Save the precipitate and discard the supernatant solution.
- Prepare a hot water bath by adding tap water to a 250 mL beaker. Add water until the beaker is about one half full. Assemble a stand, ring clamp and wire gauze to heat the water over the Bunsen burner until it is boiling. Add 2.0 mL of deionized water to the test tube containing the clean precipitate and place it in the hot water bath. Periodically stir the mixture in the test tube for 3-4 minutes.
- Remove the test tube from the hot water bath and immediately centrifuge the hot mixture. Carefully decant the hot supernatant solution into another small test tube. Save both the precipitate and the supernatant solutions for further study.
- In order to confirm the presence of \(\ce{Pb^{2+}}\), add 1 drop of 6 M \(\ce{HC2H3O2}\) to the supernatant solution obtained in Step 8. Next add 3-4 drops of 1 M \(\ce{K2CrO4}\). The formation of a bright yellow precipitate is confirmation of the presence of the \(\ce{Pb^{2+}}\) ion.
Separation and Identification of \(\ce{Hg2^{2+}}\)
- Add 10-12 drops of 6 M \(\ce{NH3}\) to the precipitate from Step 8, and mix thoroughly with your stirring rod.
- The appearance of a gray solid is confirmation of the presence of \(\ce{Hg2^{2+}}\). Centrifuge the mixture and decant the supernatant solution into another small test tube. Save the supernatant solution since it now contains silver in the form of the aqueous \(\ce{Ag(NH3)2^{+}}\) ion.
Identification of \(\ce{Ag^{+}}\)
- To the solution obtained in Step 11, slowly add 6 M \(\ce{HNO3}\) until the solution is acidic to litmus paper. The acidity can be tested by dipping a stirring rod into the solution and then touching it (with a drop of solution) to a piece of blue litmus paper resting on a clean, dry watch glass. If the solution is acidic it will turn blue litmus paper red or it will have no effect on red litmus paper. Formation of a white precipitate of \(\ce{AgCl}\) in the acidic solution is confirmation of the presence of \(\ce{Ag^{+}}\).
Part B: Analysis and Identification of Group I Cations in an Unknown Sample
- Obtain a test tube which contains a mixture of Group I cations. Record the ID Code of the sample on your Report Form
- Pour 1.0 mL of the above mixture into a second small test tube and then add 2 drops of 6 M \(\ce{HCl}\) to this test tube
- Continue following the same procedure used to analyze the known mixture of ions (Part A, Steps 3 – 13). Note that you may observe either a positive or a negative result when you test for the presence of each ion depending upon whether or not the ion is present in your unknown sample
- On your Report Form, construct a flow chart similar to the one shown in the Background section of this lab. Indicate on the flow chart whether the test for each ion is positive or negative. Then, in the space provided, indicate which ions are present in your unknown sample
Lab Report: Qualitative Analysis of Group I Cations
Name: ____________________________ Lab Partner: ________________________
Date: ________________________ Lab Section: __________________
In the space provided below construct a flow cart for the analysis of your unknown. Indicate on the flow chart whether the test for each ion is positive or negative:
Unknown ID number: __________________
Ions present in your unknown: ____________________________________
Pre-Laboratory Assignment: Qualitative Analysis of Group I Cations
- In order to identify \(\ce{Ag^{+}}\), the solution must be acidified before a precipitate can form. Why?
2. A solution may contain one or more of the Group I cations. A white precipitate forms when 6 M \(\ce{HCl}\) is added to the solution. The precipitate is insoluble in hot water. The precipitate is then found to be completely soluble in 6 M \(\ce{NH3}\). Indicate whether each of the following cations is present, undetermined, or absent.
- \(\ce{Pb^{2+}}\)_____________________
- \(\ce{Hg2^{2+}}\)_____________________
- \(\ce{Ag^{+}}\)______________________
Explain.