7: Qualitative Analysis of Group III Ions (Experiment)
- Page ID
- 95993
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To follow a classic qualitative analysis scheme to separate and identify the ions in a known mixture of Group III cations
- To apply this scheme to identify the Group III cations present in an unknown sample.
Cations are typically divided into Groups, where each group shares a common reagent that can be used for selective precipitation. In an earlier lab you performed a qualitative analysis of the Group I cations, all of which formed insoluble chlorides upon the addition of HCl (aq). In today’s lab you will analyze solutions of the Group III cations, which include \(\ce{Cr^{3+}}\), \(\ce{Al^{3+}}\), \(\ce{Fe^{3+}}\), and \(\ce{Ni^{2+}}\). All the Group III cations form insoluble sulfides or hydroxides in a basic solution saturated with \(\ce{H2S}\). The procedure will be similar to the previous Group I analysis experiment in that you will first prepare and analyze a known mixture of all four cations as a positive control. You will then analyze an unknown sample containing one, two, three or all four of the Group III cations. Your objective will be to identify which Group III cation(s) are present in your unknown sample.
Remember that in qualitative analysis the ions in a given group are first separated from each other, and then a characteristic test is performed for each ion in order to confirm the presence of that ion.
Separation and Confirmation of Group III Cations
An aqueous mixture of \(\ce{Cr^{3+}}\), \(\ce{Al^{3+}}\), \(\ce{Fe^{3+}}\), and \(\ce{Ni^{2+}}\) is first treated with a mixture of \(\ce{NaOH}\) and \(\ce{NaOCl}\) solutions. This causes the iron and nickel cations to precipitate out as hydroxide salts, while the chromium and aluminum cations remain in solution:
\[\ce{Fe^{3+} (aq) + 3 OH^{-} (aq) -> Fe(OH)3 (s) } \label{1}\]
\[\ce{Ni^{2+} (aq) + 2 OH^{-} (aq) -> Ni(OH)2 (s) } \label{2}\]
\[\ce{2 Cr^{3+} (aq) + 3 OCl^{-} (aq) + 10 OH^{-} (aq) -> 2 CrO4^{2-} (aq) + 3 Cl^{-} (aq) + 5 H2O (l) } \label{3}\]
\[\ce{Al^{3+} (aq) + 4 OH^{-} (aq) -> Al(OH)4^{-} (aq) }\label{4}\]
Note in the last two equations above that \(\ce{Cr^{3+}}\) is oxidized to the soluble \(\ce{CrO4^{2-}}\) ion while \(\ce{Al^{3+}}\) forms a soluble complex ion with \(\ce{OH^{-}}\). Neither iron nor nickel form hydroxo-complex ions and therefore precipitate out as solids. The resulting mixture is centrifuged and then decanted, separating the solids (\(\ce{Fe(OH)3}\) and \(\ce{Ni(OH)2}\)) from the supernatant solution (containing \(\ce{CrO4^{2-}}\) and \(\ce{Al(OH)4^{-}}\)).
In order to separate the chromium ions from the aluminum ions in the aqueous supernatant solution, the solution is first acidified in order to destroy the aluminum hydroxo-complex ion:
\[\ce{Al(OH)4^{-} (aq) + 4 H^{+} (aq) -> Al^{3+} (aq) + 4 H2O (l) }\label{5}\]
Next the solution is made just basic enough to precipitate out the aluminum as its hydroxide salt, but not so basic that it would remain in solution as the hydroxo-complex ion. This is accomplished by slowly adding aqueous ammonia:
\[\ce{Al^{3+} (aq) + 3 NH3 (aq) + 3 H2O (l) -> Al(OH)3 (s) + 3 NH4^{+} (aq) } \label{6}\]
The supernatant solution containing \(\ce{CrO4^{2-}}\) can then be decanted from the white gelatinous precipitate which is the solid \(\ce{Al(OH)3}\). A positive confirmation for \(\ce{Al^{3+}}\) is accomplished by dissolving the solid precipitate in acetic acid and adding the reagent catechol violet, which reacts with \(\ce{Al^{3+}}\) to produce a blue solution.
In order to confirm the presence of chromium in the supernatant solution, aqueous BaCl2 is added to it. This results in the formation of a finely divided, pale yellow precipitate of barium chromate:
\[\ce{Ba^{2+} (aq) + CrO4^{2-} (aq) -> BaCrO4 (s) } \label{7}\]
Recall that a mixture of solid precipitates of \(\ce{Fe(OH)3}\) and \(\ce{Ni(OH)2}\) is collected at the beginning of this analysis scheme. The next step is to separate them. First the hydroxide precipitates are dissolved in nitric acid:
\[\ce{Ni(OH)2 (s) + 2 H^{+} (aq) -> Ni^{2+} (aq) + 2 H2O (l) } \label{8}\]
\[\ce{Fe(OH)3 (s) + 3 H^{+} (aq) -> Fe^{3+} (aq) + 3 H2O (l) } \label{9}\]
The acidified solution is then made basic by adding aqueous ammonia. Once the solution has become basic, the excess ammonia will react with \(\ce{Ni^{2+}}\) to form an aqueous complex ion while the \(\ce{Fe^{3+}}\) will precipitate out once more as red \(\ce{Fe(OH)3}\) since \(\ce{Fe^{3+}}\) does not typically form complex ions with \(\ce{NH3}\):
\[\ce{Ni^{2+} (aq) + 6 NH3 (aq) -> Ni(NH3)6^{2+} (aq) } \label{10}\]
\[\ce{Fe^{3+} (aq) + 3 NH3 (aq) + 3 H2O (l) -> Fe(OH)3 (s) + 3 NH4^{+} (aq) } \label{11}\]
The presence of \(\ce{Ni^{2+}}\) in the aqueous solution is confirmed by adding dimethylglyoxime (\(\ce{C4H8N2O2}\)) resulting in the formation of a rose red precipitate:
\[\ce{Ni^{2+} (aq) + 2 C4H8N2O2 (aq) -> Ni(C4H7N2O2)2 (s) + 2 H^{+} (aq) } \label{12}\]
In order to confirm the presence of \(\ce{Fe^{3+}}\), the red \(\ce{Fe(OH)3}\) precipitate is dissolved in \(\ce{HCl}\) (aq), and then \(\ce{KSCN}\) (aq) is added to the solution. A positive result is the formation of a dark red solution indicating the presence of \(\ce{FeSCN^{2+}}\) (aq):
\[\ce{Fe^{3+} (aq) + SCN^{-} (aq) -> FeSCN^{2+} (aq) } \label{13}\]
The entire analysis scheme is represented in abbreviated form using the follwing flow chart:
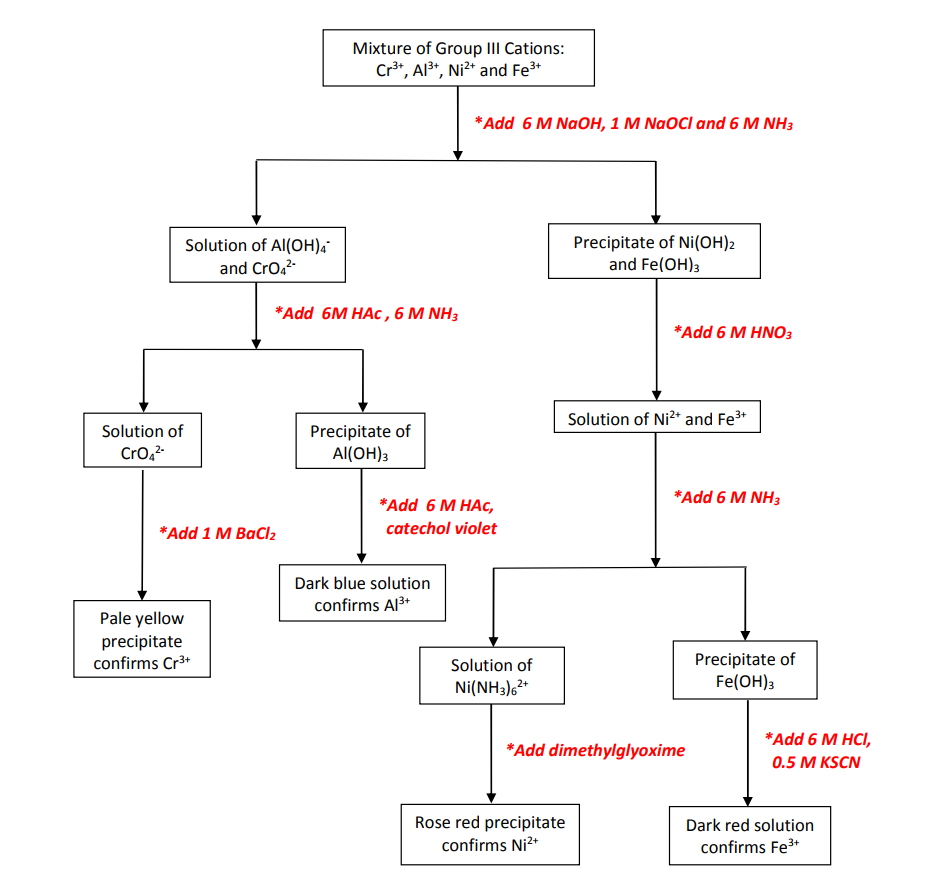
Figure 1: GROUP III CATIONS ANALYSIS SCHEME
Procedure
Chemicals
0.1 M \(\ce{Cr(NO3)3}\) (aq), 0.1 M \(\ce{Al(NO3)3}\) (aq), 0.1 M \(\ce{Ni(NO3)2}\) (aq), 0.1 M \(\ce{Fe(NO3)3}\) (aq), 6 M \(\ce{HCl}\) (aq), 6 M \(\ce{HNO3}\) (aq), 6 M acetic acid, 6 M \(\ce{NaOH}\) (aq), 6 M \(\ce{NH3}\) (aq), 1 M \(\ce{NaOCl}\) (aq), 1 M \(\ce{BaCl2}\) (aq), 0.5 M \(\ce{KSCN}\) (aq), catechol violet, dimethylglyoxime.
Equipment
12 small test tubes, 30-mL beaker, beaker tongs, Bunsen burner, 10-mL graduated cylinder, glass stirring rod, small watch glass, dropper pipets, red and blue litmus paper, centrifuge, wash bottle filled with deionized water, 150-mL beaker (for waste solutions).
For the hot water bath: 250-mL beaker, stand, ring clamp, wire gauze
- The PPE for this lab includes safety goggles, lab coat and nitrile gloves.
- Exercise appropriate caution when using all concentrated acids and bases in this lab as they can cause serious chemical burns to your skin. If any acid comes into contact with your skin or eyes, immediately rinse the affected areas with copious amounts of water for 15 minutes, and inform your instructor.
Waste Disposal
All the waste chemicals generated by this lab are toxic and must be disposed of in the hazardous-waste container in the fume hood. Rinse all glassware directly into the waste container using a wash bottle.
Any unused portion of the unknown sample should be discarded in the hazardous-waste container. Rinse the test tube and return it to the instructor’s bench at the end of lab.
General Instructions
Similar to last week’s procedure, you will analyze a known mixture of all four Group III cations as a positive control experiment. You will then analyze an unknown sample in order to determine which Group III cations are contained within the sample.
- Be sure to label all tubes and solutions because they will accumulate rapidly, and it is very easy to get tubes and/or solutions mixed up.
- Make sure all glassware is thoroughly cleaned and rinsed with deionized water between uses to avoid cross-contamination.
- The centrifuge must be balanced before each use. Balancing is done by placing a test tube containing water opposite to the test tube containing the precipitate in the centrifuge. The two test tubes should have approximately equal weights.
- The pH of a solution is tested by dipping a stirring rod into the solution and then touching it to a piece of litmus paper resting on a clean, dry watch glass. If a solution is acidic it will turn blue litmus paper red. If a solution is basic it will turn red litmus paper blue.
Part A: Analysis of Known Mixture of Group III Cations – A Positive Control Experiment
Preparation of Known Solution and Separation of \(\ce{Cr^{3+}}\) and \(\ce{Al^{3+}}\) from \(\ce{Fe^{3+}}\) and \(\ce{Ni^{2+}}\)
- Prepare a mixture of Group III cations by adding 1.0 mL of each of the following 0.1 M aqueous solutions to a small test tube: \(\ce{Cr(NO3)3}\), \(\ce{Al(NO3)3}\), \(\ce{Fe(NO3)3}\), and \(\ce{Ni(NO3)2}\). Transfer 2.0 mL of this mixture to a 30-mL beaker and use it for your positive control experiment.
- Add 2 mL of 6 M \(\ce{NaOH}\) to the 30-mL beaker containing your positive control solution. Boil the solution very gently (use a pair of beaker tongs to hold the beaker) over an open Bunsen burner flame for about one minute. Note that the time to boil is variable and depends on the volume of solution you have. Do not boil the solution to dryness. If you boil the solution to dryness, then you must repeat this step again.
- Remove the beaker from the flame and slowly add 2 mL of 1 M \(\ce{NaClO}\). Swirl the beaker for 30 seconds using your beaker tongs, then gently boil the mixture for about one minute.
- Again, remove the beaker from the flame and add sixteen drops of 6M \(\ce{NH3}\). Swirl the mixture, then gently boil for another minute.
- Transfer the mixture from the beaker into a small test tube and centrifuge in order to separate the solid from the solution. Decant the supernatant solution (containing the chromium and the aluminum) into another test tube and save the solid (containing the nickel and the iron). Save this supernatant solution for further analysis. It contains the aluminum and chromium cations.
- Wash the solid by adding 4 mL of water and twenty drops of 6 M \(\ce{NaOH}\). Stir vigorously then centrifuge the mixture. Decant the supernatant solution into your “waste” beaker and discard this solution.
- Add 2 mL of 6 M \(\ce{HNO3}\) to the washed solid. Save the contents of this test tube for further analysis. It contains the iron and nickel cations.
Separation of \(\ce{Al^{3+}}\) from \(\ce{Cr^{3+}}\)
- Slowly add 6 M acetic acid to the supernatant solution saved from Step 5 until it is acidic (test with blue litmus paper).
- Add 6 M \(\ce{NH3}\) one drop at a time until the solution just becomes basic again (test with red litmus paper). Now add twenty additional drops of excess \(\ce{NH3}\). At this point a translucent, gelatinous precipitate of \(\ce{Al(OH)3}\) should appear in the clear (or pale yellow) solution.
- Stir the mixture for about a minute to allow the system to reach equilibrium. Centrifuge the mixture, then decant the supernatant solution into another small test tube. This solution must be saved for further tests.
- To wash the collected solid, first add 6 mL of deionized water to it. Then place the test tube with the solid (and water) in a hot water bath for about two minutes while occasionally stirring the mixture. Check with your instructor if you are unsure about how to set up a hot water bath. Finally, centrifuge this mixture and decant the supernatant solution into your “waste” beaker. Discard the solution and save the solid.
Confirmation of \(\ce{Al^{3+}}\)
- To the solid from Step 11 add four drops of 6 M acetic acid and 6 mL of water. Stir in order to dissolve the solid. Finally, add four drops of catechol violet to the resulting solution. The appearance of a blue solution confirms the presence of \(\ce{Al^{3+}}\) in the original solution.
Confirmation of \(\ce{Cr^{3+}}\)
- To the decanted solution from Step 10, add twelve drops of 1 M \(\ce{BaCl2}\). The formation of a very fine, pale yellow precipitate (\(\ce{BaCrO4}\)) confirms the presence of \(\ce{Cr^{3+}}\) in the original solution.
Separation of \(\ce{Fe^{3+}}\) from \(\ce{Ni^{2+}}\)
- If the precipitate from Step 7 is not completely dissolved, stir it until it is completely dissolved. If necessary, place the test tube in a hot water bath in order to completely dissolve the precipitate.
- Slowly add 6 M \(\ce{NH3}\) to the solution until it is basic (test with red litmus paper). You should see a red-brown precipitate of \(\ce{Fe(OH)3}\) appear when the solution turns basic.
- Add an additional 20 drops of 6 M \(\ce{NH3}\) and stir to mix well. At this point the nickel should be dissolved in the solution as \(\ce{Ni(NH3)6}\).
- Centrifuge the mixture and decant the supernatant solution into another test tube. Save both the solid and solution.
Confirmation of \(\ce{Ni^{2+}}\)
- Add eight drops of dimethylglyoxime to the solution saved in Step 17. The appearance of a rose-red precipitate confirms the presence of \(\ce{Ni^{2+}}\) in the original solution.
Confirmation of \(\ce{Fe^{3+}}\)
- To the precipitate saved in Step 17 add twelve drops of 6 M \(\ce{HCl}\). Next, add 4 mL of deionized water and stir well until the precipitate is completely dissolved. Finally, add four drops of 0.5 M \(\ce{KSCN}\). The formation of a dark red solution confirms the presence of \(\ce{Fe^{3+}}\) in the original solution.
Part B: Analysis and Identification of Group III Cations in an Unknown Sample
- Obtain a test tube which contains a mixture of Group III cations. Record the ID Code of the sample on your Report Form.
- Transfer 2.0 mL of the above mixture into a 30-mL beaker and repeat the procedure used in Part A.
- On your Report Form, construct a flow chart similar to the one shown in the Background. Indicate on the flow chart whether the test for each ion is positive or negative. Then, in space provided, indicate which ions are present in your unknown sample.
Lab Report: Qualitative Analysis of Group III Cations
Name: ____________________________ Date: ________________________
Lab Partner: ________________________ Lab Section: __________________
In the space provided below construct a flow cart for the analysis of your unknown. Indicate on the flow chart whether the test for each ion is positive or negative:
Unknown ID number: __________________
Ions present in your unknown: ____________________________________
Pre-laboratory Assignment: Qualitative Analysis of Group III Cations
- Given the very low value of \(K_{sp}\) for \(\ce{Cr(OH)3}\), a precipitate of \(\ce{Cr(OH)3}\) would be expected if only 6 M \(\ce{NaOH}\) were added to the mixture of Group III cations in the first step of the procedure. Explain how and why the chromium remains in solution.
- A solution may contain one or more of the Group III cations. When this solution is combined with \(\ce{NaOH}\) (aq), \(\ce{NaOCl}\) (aq) and \(\ce{NH3}\) (aq) only a colorless solution is obtained with no precipitate evident. Indicate whether each of the following cations is present, absent or undetermined.
- \(\ce{Cr^{3+}}\) _____________________
- \(\ce{Al^{3+}}\) _____________________
- \(\ce{Fe^{3+}}\) _____________________
- \(\ce{Ni^{2+}}\) _____________________
Explain.


