Glass Density Evidence
- Page ID
- 50013
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Sometimes identifying one type of glass or glass fragment at a crime scene may be critical in solving a crime, but the largest database [1] includes 304,107 glass compositions, so identifying one of them can be daunting. Although chemists can identify glass by more conclusive methods involving elemental analysis, density may still be used as a screening method, as FBI documents indicate FBI Glass Density

Density can be used to identify smashed glass at the scene of a crime.
Densities of dozens of glasses are known [1]. The most common type of glass is ordinary window or bottle glass. It's called soda-lime glass because it is made of sodium carbonate (Na2CO3) and CaO (calcium oxide or lime) in addition to silica (sand or quartz, (SiO2). Pyrex (the Corning brand of borosilicate glass, about 70% silica, 10% boron oxide (B2O3), 8% sodium oxide, 8% potassium oxide, and 1% calcium oxide).
Glass densities are determined by an ASTM standard method involving flotation in liquids [3], but the density of glass fragments can also be measured by water displacement, as described below.
The ASTM floatation method is interesting. A layer of "heavy" bromoform is first added to a cylinder. It has a density of 2.889 g/cm3 at 15 °C, so most common glasses will float on its surface. Next, a layer of 20% ethanol in bromoform is carefully floated on the bromoform. Ethanol is light, having a density of 0.789 g/cm3, so when it's mixed with bromoform, the resulting solution is just a bit denser than bromoform. Layers of 40%, 60%, and 80% ethanol in bromoform, followed by 100% ethanol are added, and the layers are allowed to sit overnight. They diffuse into one another, giving a gradually changing density from 0.789 to 2.889 g/cm3. When glass pieces are added, they float at the level where the density matches. A density gradient column can be created with sugar solutions in dyed water [4], as shown in the figure.
Density Gradient
The terms heavy and light are commonly used in two different ways. We refer to weight when we say that an adult is heavier than a child. On the other hand, something else is alluded to when we say that flint optical glass is heavier than ordinary bottle glass. A small shard of flint would obviously weigh less than a gallon glass jug, but flint is heavier in the sense that a piece of given size weighs more than the same-size piece of bottle glass.
What we are actually comparing is the mass per unit volume, that is, the density. In order to determine these densities, we might weigh a cubic centimeter of each type of glass. If the flint sample weighed 7.2 g and the bottle glass 2.4 g, we could describe the density of flint as 7.2 g cm–3 and that of bottle glass as 2.4 g cm–3. (Note that the negative exponent in the units cubic centimeters indicates a reciprocal. Thus 1 cm–3 = 1/cm3 and the units for our densities could be written as 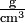 , g/cm3, or g cm–3. In each case the units are read as grams per cubic centimeter, the per indicating division.) We often abbreviate "cm3" as "cc", and 1 cm3 = 1 mL exactly by definition.
, g/cm3, or g cm–3. In each case the units are read as grams per cubic centimeter, the per indicating division.) We often abbreviate "cm3" as "cc", and 1 cm3 = 1 mL exactly by definition.
| Glass Type | Density/g/cm3 |
|---|---|
| sand | 1.52 |
| fused silica (96%) | 2.18 |
| Corning Vycor® 7907 UV-Blocking Glass | 2.21 |
| Pyrex(R) | 2.23 |
| borosilicate glass | 2.4 |
| ordinary bottle | ~2.4-2.8 |
| ordinary window | ~2.4-2.8 |
| Corning 0211 Zinc Borosilicate Glass | 2.53 |
| Corning 1724 Aluminosilicate Crushed/Powdered Glass | 2.64 |
| crown glass | 2.8 |
| Corning 0159 Lead Barium Crushed/Powdered Glass | 3.37 |
| lead crystal | 3.1 |
| Corning 8870 Potash Lead Crushed Glass | 4.28 |
| densest flint optical | 7.2 |
| Densities of many more materials are easily found |
In general it is not necessary to weigh exactly 1 cm3 of a material in order to determine its density. We simply measure mass and volume and divide volume into mass:
\[\text{Density} = \dfrac{\text{mass}} {\text{volume}}\]
or
\[\rho = \dfrac{\text{m}} {\text{V}}\]
where ρ = density m = mass V = volume
Example \(\PageIndex{1}\): Density Calculation
Calculate the density of (a) a piece of glass shard whose mass is 37.42 g and which, when submerged, increases the water level in a graduated cylinder by 13.9 ml; (b) a glass cylinder of mass 25.07 g, radius 0.750 cm, and height 5.25 cm.
Solution
a) Since the submerged metal displaces its own volume,
\[\text{Density} =\rho = \dfrac{\text{m}} {\text{V}} = \dfrac{37.42 g} {13.9 mL} = \text {2.69 g/mL or 2.69 g mL}^{-1}\]
b) The volume of the cylinder must be calculated first, using the formula
\[\text{V} = {\pi} r^{2} h = 3.142 × \text{(0.750 cm)}^{2} * 5.25 \text{cm} = \text{9.278 718 8 cm}^{3}\]
Then \[\rho = \dfrac{\text{m}} {\text{V}} = \dfrac{25.07g} {9.278 718 8cm^{3}}\]
\[= \text{2.70} \dfrac{\text{g}}{\text{cm}^3}\]
Note
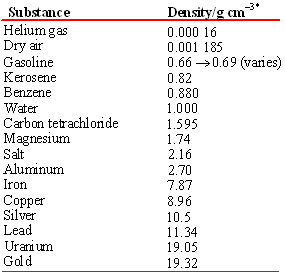
Note that unlike mass or volume, the density of a substance is independent of the size of the sample. Thus density is a property by which one substance can be distinguished from another. A sample of glass can be broken or adjusted to have any mass we choose, but its density will always be 2.70 g/cm3 at 20°C, so the forensic sample can be used to identify the glass bottle from which it was broken. The densities of some common pure substances are listed in the Table.
Tables and graphs are designed to provide a maximum of information in a minimum of space. When a physical quantity (number × units) is involved, it is wasteful to keep repeating the same units. Therefore it is conventional to use pure numbers in a table or along the axes of a graph. A pure number can be obtained from a quantity if we divide by appropriate units. For example, when divided by the units gram per cubic centimeter, the density of aluminum becomes a pure number 2.70:
\[\dfrac{\text{Density of aluminum}} {\text{1 g cm}^{-3}} = \dfrac{\text{2.70 g cm}^{-3}} {\text{1 g cm}^{-3}} = 2.70\]
TABLE \(\PageIndex{2}\) Density of Several Substances at 20°C.
Therefore, a column in a table or the axis of a graph is conveniently labeled in the following form:
\[\dfrac{\text{Quantity}}{\text{units}}\]
This indicates the units that must be divided into the quantity to yield the pure number in the table or on the axis. This has been done in the second column of Table 1.4.
Converting Densities
In our exploration of Density, notice that chemists may express densities differently depending on the subject. The density of pure substances may be expressed in kg/m3 in some journals which insist on strict compliance with SI units; densities of soils may be expressed in lb/ft3 in some agricultural or geological tables; the density of a cell may be expressed in mg/µL; and other units are in common use. It is easy to transform densities from one set of units to another, by multiplying the original quantity by one or more unity factors:
Example \(\PageIndex{2}\): Density Conversion
Convert the density of water, 1 g/cm3 to (a) lb/cm3 and (b) lb/ft3
a. The equality 454 g = 1 lb can be used to write two unity factors,
\(\dfrac{\text{454 g}} {\text{1 lb}}\) or \(\dfrac{\text{1 lb}} {\text{454}}\)
The given density can be multiplied by one of the unity factors to get the desired result. The correct conversion factor is chosen so that the units cancel:
\(\dfrac{\text{1 g}} {\text{cm}^{3}}* \dfrac{\text{1 lb}} {\text{454 g}} = \text{0.002203} \dfrac{\text{lb}} {\text{cm}^{3}}\)
b. Similarly, the equalities 2.54 cm = 1 inch, and 12 inches = 1 ft can be use to write the unity factors:
\(\dfrac{\text{2.54 cm}} {\text{1 in}}\), \(\dfrac{\text{1 in}} {\text{2.54 cm}}\), \(\dfrac{\text{12 in}} {\text{1 ft}}\) and \(\dfrac{\text{1 ft}} {\text{12 in}}\)
In order to convert the cm3 in the denominator of 0.002203 \(\dfrac{lb} {cm^{3}}\) to in3, we need to multiply by the appropriate unity factor three times, or by the cube of the unity factor:
\(\text{0.002203} \dfrac{\text{g}} {\text{cm}^{3}}\) x \(\dfrac{\text{2.54 cm}} {\text{1 in}}\) x \(\dfrac{\text{2.54 cm}} {\text{1 in}}\) x \(\dfrac{\text{2.54 cm}} {\text{1 in}}\)
or
\(\text{0.002203} \dfrac{\text{g}} {\text{cm}^{3}}\) x \((\dfrac{\text{2.54 cm}} {\text{1in}})^{3} = \text{0.0361} \dfrac{\text{lb}}{\text{in}^3}\)
This can then be converted to lb/ft3:
\(\text{0.0361} \dfrac{\text{lb}} {\text{in}^{3}}\) x \(\dfrac{\text{12 in}} {\text{1 ft}})^{3} = \text{62.4} \dfrac{\text{lb}}{\text{ft}^3}\)
Note
It is important to notice that we have used conversion factors to convert from one unit to another unit of the same paramter.
From ChemPRIME: 1.8: Density
References
- www.sciglass.info/
- www.fbi.gov/hq/lab/fsc/backis...standards8.htm
- www.americanglassresearch.com...Density%20Oils
- http://chemistry.about.com/od/chemistrydemonstrations/ht/rainbowinaglass.htm
- Red food color in 24% sugar solution; Yellow: 16% sugar; Green: 12% sugar; Blue: 8% sugar
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


