Radon and Nuclear Chemistry
- Page ID
- 50925
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Radon is a colorless odorless gas that is a radioactive decay product of uranium. The half life of a radioactive sample is useful in determining how harmful a radioactive isotope may be. In 1904 Ernest Rutherford proposed the concept of half-life to describe the random process of radioactive decay. Half life expresses the time within which any particular radioactive atom has a 50:50 chance of undergoing radioactive decay. No one can say for sure when a particular nucleus will decay but one can predict how many in a given sample will decay over time. A more recent definition states the radioactive half-life for a given radioisotope is the time for half the radioactive nuclei in any sample to undergo radioactive decay. After two half-lives, there will be one fourth the original sample, after three half-lives one eighth the original sample, and so forth. So if you have 10 grams of a radioactive element, after one half-life there will be 5 grams of the radioactive element left. After another half-life, there will be 2.5 g of the original element left, after another half-life, 1.25 g will be left. The equation for half-life calculations is as follows:
\[\text{A}_E = \text{A}_0 * \text{0.5}^{t/t_{1/2}}\]
• AE is the amount of substance left
• A0 is the original amount of substance
• t is the elapsed time
• t1/2 is the half-life of the substance
An example problem is if you originally had 157 grams of carbon-14 and the half-life of carbon-14 is 5730 years, how much would there be after 2000 years?
\[\text{A}_E = \text{157} * \text{0.5}^{2000/5730}\]
There would be 123 grams left.
Radon's most stable isotope, radon-222, has a half-life of about 3.8 days. This seems to be a relatively short half life. So why is there so much concern about radon in homes? To understand completely we should consider the types of radioactive decay.
There are three types of natural radioactive decay termed alpha, beta, and gamma. An alpha particle is composed of two protons and two neutrons; it is identical in composition to the nucleus of a helium atom. Alpha particles have no electrons so they have a +2 electrical charge. Alpha particles have a relatively large mass which makes them relatively easy to stop outside of the body but the electrical charge and energy of an alpha particle can cause damage to tissues over a short distance. Beta particles are fast moving electrons emitted from the nucleus during radioactive decay. Since they are much lighter and travel at high velocities beta particles have greater penetrating power than alpha particles. However, they are not as damaging to human tissue. Alpha and beta decay often leave nuclei in an excited state described as metastable. Energy from isotopes in metastable states is released as gamma rays. These are high energy electromagnetic radiation that do not have mass or charge. Of the three forms of radioactive decay gamma rays are the most penetrating but under most circumstances they cause the least amount of tissue damage over comparable distances. This is because tissue damage is related to the extent of ionization created by the radiation expressed as the number of ionizations within each unit of tissue. The relative penetrating power of the three types of nuclear radiation are shown below.
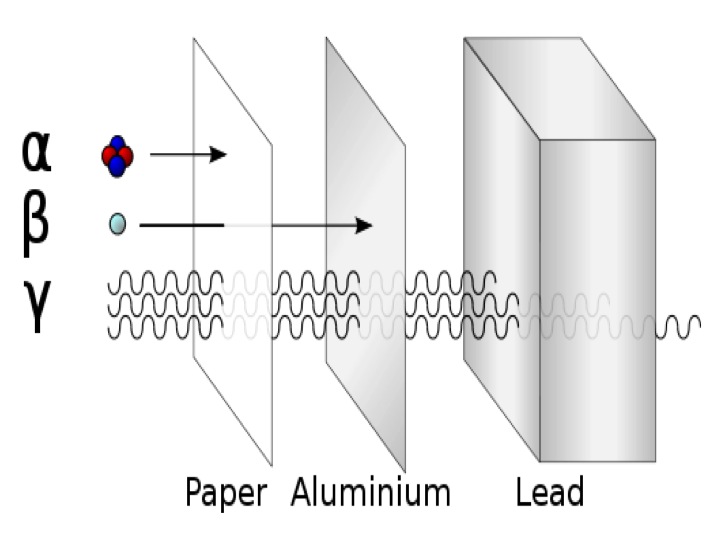
Figure \(\PageIndex{1}\) The relative penetrating power of three types of nuclear radiation
The following chart shows the radioactive decay chain with half-life for uranium 238. Notice that uranium has a very long half life (4.5 billion years). Radon is formed as part of the normal radioactive decay chain of uranium. Uranium has been around since the earth was formed and the most common isotope has a very long half-life. Uranium and thorium, radium, and thus radon, will continue to occur for millions of years at about the same concentrations as they do now.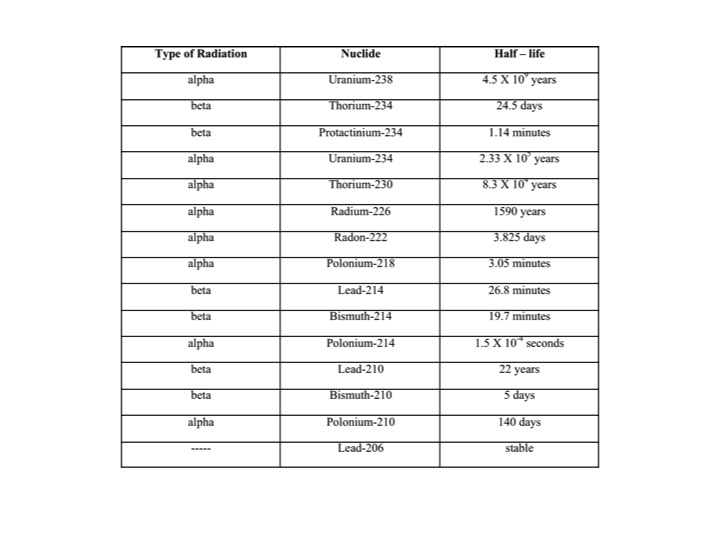
Nuclear reactions can be written for each of the steps. Below is the nuclear reaction for the step where radon-222 undergoes alpha decay to form polonium-218.
\[{}_{\text{86}}^{\text{222}}\text{Rn}\to \text{ }{}_{\text{84}}^{\text{218}}\text{Po + }{}_{\text{2}}^{\text{4}}\text{He}\]
Alpha decay usually isn't considered to be a great radiological hazard since the alpha particles produced by the decay are easily stopped and with a short half-life one would think that radon would not be a big threat. The most serious danger of radon gas results from subsequent reactions. Radon radioactively decays to produce radioactive isotopes of polonium, bismuth, and lead. These decay products are solids and cannot be exhaled once inside the body. Outside of the body these decay products can stick to surfaces such as dust particles in the air. If contaminated dust is inhaled these particles can stick to the airways of the lung. Furthermore, these decay products are also alpha particle emitters. The alpha particles emitted in the decay of radon daughters (polonium, bismuth, and lead), in spite of their poor penetrating power, can reach these very sensitive cells because they are deposited so close to them. To make matters worse, alpha particles are much more efficient than other types of radiation for inducing cancer. The very fact that they are not penetrating means that they dump a lot of their energy into each of the biological cells they pass through, and this large release of energy into a single cell is just what is needed to initiate a cancer. As a result, an alpha particle is a hundred times more likely to cause cancer than other types of radiation, if it can reach the target cells. Since radon is a gas our breathing processes allow the alpha particles from radon daughters to reach these cells.
From ChemPRIME: 19.0: Prelude to Nuclear Chemistry
Reference:
Freeman, W. H. (2002). Chemistry in the community. (4 ed., pp. 428-443).
New York: American Chemical Society.
http://education.jlab.org/itselemental/ele086.html
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radon.html
library.thinkquest.org/10429/...ar/nuclear.htm
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


