Cyclopamine and Cancer Prevention
- Page ID
- 50831
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One-eyed humans may be figures of fiction presented in tales like the Odyssey, but cyclopian sheep are real. In 1957, the U.S Department of Agriculture conducted an investigation in the mountains of Idaho to find out why nearly 40% of some herders' sheep were giving birth to lambs with a single eye. A decade-long study identified consumption of an organic compound in the wild corn lily as the cause. The plant chemical was found to leave the mother unharmed but stunted the development of a gene in the embryos, and was dubbed cyclopamine[1]. Researchers found that ewes that consumed the corn lily on their 14th day of gestation gave birth to cyclopian lambs. Using this information, herders were able to control the grazing habits of their flocks and prevent the defect.

As its name suggests, cyclopamine is part of the amine family, an organic compound group that are essentially derivatives of the basic molecule ammonia, NH3. An amine is derived from ammonia by replacing any number of the hydrogen atoms with another group, which may be a pure hydrocarbon or contain other functional groups. Since the potential substituents are essentially limitless, amines can be extremely complex molecules, as exemplified by cyclopamine's structure.
Amines can be separated into three different groups, depending on the number of hydrogen atoms replaced. A primary amine replaces one hydrogen atom, a secondary amine replaces two hydrogens, and a tertiary amine replaces three. The following image shows examples of these categories:
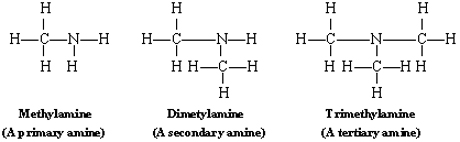
Example \(\PageIndex{1}\): Melting and Boiling Points of Amines
Of the three types of amines, which do you expect to have the highest melting and boiling points (when comparing similarly-sized molecules)?
Answer:
Each type of amine has different physical and chemical properties. Primary amines contain more hydrogen atoms, thus more N-H bonds, and are thus the most likely to exhibit hydrogen bonding, resulting in higher melting and boiling points.
Amines have many more differences in physical and chemical properties. For example, tertiary amines are saturated and do not react with many compounds that a primary or secondary amine would.
Example \(\PageIndex{2}\): Cyclopamine
From the structure above, determine whether cyclopamine is a primary, secondary, or tertiary amine.
Answer:
In order to determine the classification of cyclopamine, we must identify what groups are bonded to its nitrogen atom, which is in the upper right area of the given projected structure. We know that there can be only three group total, as nitrogen is stable when it has a lone pair in its valence shell. We are given one obvious N-H bond and two other bonds that continue into the main body of the molecule. Following general projection formula notation, these other two bonds must be connected to carbon atoms.
Therefore, with one N-H bond and two N-C bonds, cyclopamine is a secondary amine.
Interestingly, cyclopamine can also combat the growth of cancer, stunting the growth of tumors in much the same way they inhibit embryo development. Cyclopamine mutates lambs by inhibiting a particular gene that is crucial in the development of an embryo. The name of the gene, sonic hedgehog, serves as a sly allusion and a description of the embyro's development of small "spikes" if the gene's functions are blocked. Sonic hedgehog continues to control tissue growth throughout an organism's life and is responsible for some types of child brain cancer and white blood cell cancer. By inhibiting the sonic hedgehog pathway in mice with a controlled dose of cyclopamine, scientists hope to find a way to combat potentially deadly tumors in humans.
Amides and Amino Acids
Not all nitrogen compounds are so destructive to organic tissue. Consider the condensation reaction between an amine and a carboxylic acid, both generally organic compounds with harmful effects on the body. The product is an amide, a compound with an amine group bonded to the carbon of a carboxyl group.
Cyclopamine can be made less toxic in cancer treatments by combining it with amino acids [2].
Certain amides required for biological functions are called amino acids. Molecules produced by condensation reactions, such as amino acids, can usually form condensation polymers, huge molecules made up of a repeated chain of smaller molecules. Condensation polymers of amino acids are the proteins we know are essential for muscle and bone growth. Some amino acids are naturally produced inside the body, while others, such as lysine, are not.
There are many more varieties of nitrogen compounds that are of lesser population within the body. We have only covered nitrogen compounds which contain only single N-R bonds. Compounds with a triple bond from C to N are called nitriles, or cyanides. One probably already knows what effect a cyanide has on the human body; this is a topic for another exemplar.
From ChemPRIME: 8.18: Organic Nitrogen Compounds
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


