4: The Discovery of Fission (1938)
- Page ID
- 19569
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)From 1935-1938, Hahn, Strassmann, and Meitner in Germany identified at least 10 radioactive products resulting from the neutron bombardment of uranium, many more than Fermi’s group in Italy had observed initially. They assumed that these substances were new transuranic elements or isotopes of uranium. The Joliot-Curies had also performed this experiment and thought that they had observed thorium and actinium (elements below, not beyond, uranium in the periodic chart). These differences with Fermi’s results led both groups to continue their investigations.

Early in December 1938, Hahn and Strassmann thought that they had established the nuclear reactions that yielded the products observed by the Joliot-Curies. Presumably, isotopes of radium produced by the initial neutron bombardment of uranium decayed to thorium and actinium. To be absolutely certain, Hahn and Strassmann decided to identify the ‘radium’ isotopes by chemical means. (The chemical separation is shown below.) The suspected radioisotope of 'radium' is indicated as 'Ra'. Since radium and barium are group IIA elements, having the same chemical properties, barium was added as a carrier to facilitate the chemical isolation of the small amounts of the suspected 'radium'.
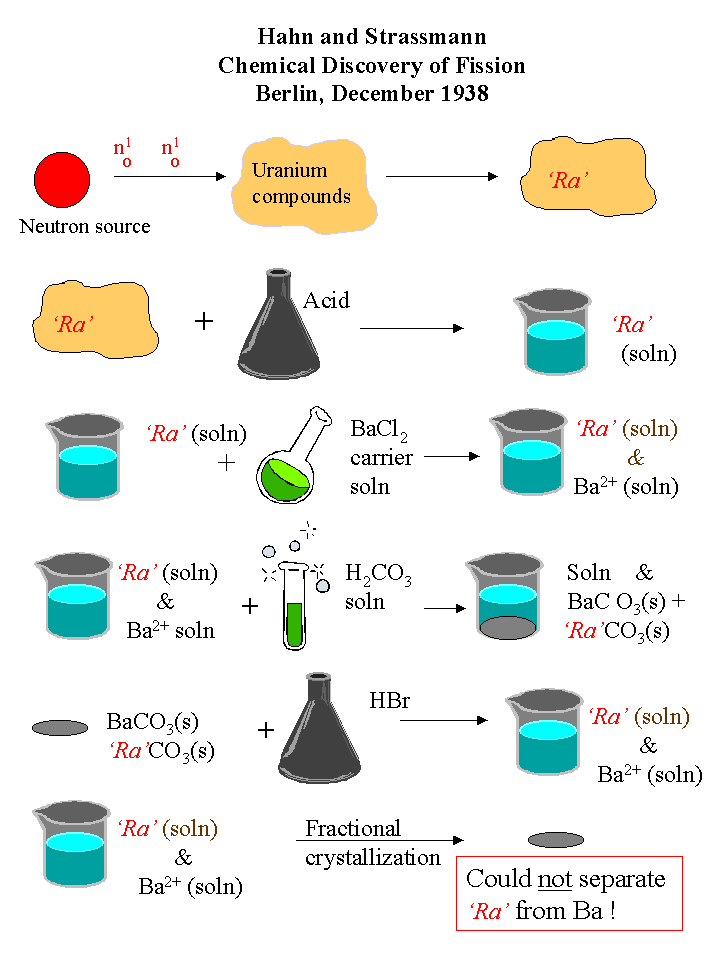
The final step in this procedure was a fractional crystallization to separate the barium carrier from the suspected 'radium'. Hahn and Strassmann were unable to separate the radioactivity of the 'Ra' from that of the barium fractions and thus confirmed that one of the products of neutron bombardment of uranium was not neighboring radium, but distant barium. Could Ida Noddack’s hypothesis be correct? Had the uranium atoms split into fragments of approximately equal mass?

Hahn and Strassmann’s laboratory apparatus
Hahn and Strassmann repeated the experiment numerous times and were never able to isolate the ‘radium’ from barium. They reported their results as follows: "As chemists, we must actually say the new particles do not behave like radium but, in fact, like barium; as nuclear physicists, we cannot make this conclusion, which is in conflict with all experience in nuclear physics." Hahn, the chemist, was reluctant to go against the ideas of respected nuclear physicists, despite clear chemical evidence of barium.
Hahn then turned to his colleague, Lise Meitner, for an explanation. Meitner was a physicist who had recently fled to Sweden to escape the Nazi regime. During Christmas 1939, Meitner and her nephew Otto Frisch, a physicist also banished from Germany, read Hahn’s letters reporting, with amazement, the barium results. As Meitner and Frisch searched for an explanation, it dawned on them that when the uranium nucleus absorbs a neutron, it might become unstable and split into two particles of approximately equal mass (e.g., barium and krypton). They used Bohr’s earlier model, which treated the nucleus as a large drop of liquid. In this model, the absorption of a neutron could cause the uranium nucleus to become unstable and divide into two smaller drops. If this division takes place, the resulting drops (nuclear fragments) would be repelled by their respective positive charges. This process was termed fission by Meitner and Frisch.

Otto Frisch (Courtesy of the American Institute of Physics)
They quickly understood that fission would create a large amount of energy, and balanced nuclear equations indicated additional neutrons would be produced. They calculated the energy associated with pushing the two positively charged nuclear fragments apart to be approximately 200 million electron volts (MeV) per uranium atom. By comparison, the most energetic chemical reactions release approximately 5 eV per atom.
From where was the energy required to separate the uranium nucleus coming? Existing data on the masses of the elements showed that the sum of the masses of the smaller product nuclei was less than the mass of uranium. Meitner used Einstein’s famous \(E = mc^2\) equation to calculate the energy associated with this mass difference (nuclear binding energy) and the energy associated with pushing the two fission fragments apart. His calculations revealed that energy equivalent to the mass difference was equal to 200 MeV! Frisch quickly returned to his laboratory at the Neils Bohr Institute in Copenhagen to experimentally verify this hypothesis.
The scientific community was excited by the discovery of this new source of energy. When Frisch presented his explanation to Bohr, Bohr is said to have struck his forehead and exclaimed, "What idiots we have all been! Oh but this is wonderful! It just must be!" Energy from fission was at least eight orders of magnitude greater than the energy released in chemical reactions involving an equal number of atoms.
Furthermore, fission raised the possibility of a nuclear chain reaction. Radioactive fission products have excess neutrons, compared with stable (nonradioactive) nuclei of the same mass number. They can eliminate the excess neutrons by beta decay, a slow process that increases the atomic number by one, or by direct neutron emission. In the latter case, the secondary neutrons may be used to produce new fissions and if a sufficient number of fissions occur, they will produce enough neutrons to sustain a chain reaction.
It was theorized that if such a chain reaction could be controlled, one would have a tremendous source of power. Scientists began to speculate on the potential of nuclear power plants. But if the chain reaction were to occur in a violent, uncontrolled manner, one would have an atomic bomb. Scientists also began to worry about the potential for nuclear weapons. It is interesting to note that the science fiction writer H. G. Wells had written about the potential of nuclear power in "The World Set Free", published in 1914.
One scientist, Leo Szilard, began to worry about the potential for nuclear weapons in the face of the growing hostility of Germany and the likelihood of massive, technically based warfare. Szilard, the Hungarian expatriate physicist, deduced that the discovery of fission would lead either Germany or the Allies to high energy weapons. He sought an audience with President Franklin Roosevelt but he was rebuffed. But Szilard got assurance that a letter from Einstein would be delivered to Roosevelt. Szilard went to Einstein, who prepared the letter shown below.


As we look forward to the early years of the new millennium we might reflect that it was the physicist Albert Einstein whom TIME magazine chose as the most important individual of the 20th century!
One final question remained concerning the fission of uranium. In 1935, Arthur Dempster, a Canadian-born chemist working at the University of Chicago, used a mass spectrometer to find a heretofore unknown isotope of mass 235 hidden in natural uranium, the overall mass of which was approximately 238. Three years later, Alfred Nier used a mass spectrometer to measure the ratio of U-235 to U-238 in natural uranium as 1:139, which meant that U-235 was present to the extent of 0.7%.
Meanwhile, at Princeton University, Niels Bohr and John Wheeler theorized that U-238 only underwent fission with fast, high-energy neutrons, while slow, thermal neutrons would produce fission only in U-235. John Dunning, a physicist working with Fermi at Columbia University, decided to test the Bohr-Wheeler theory and made a deal with Nier to obtain a few micrograms of pure U-235. Using this small sample, Dunning’s group proved the Bohr-Wheeler hypothesis. Until this discovery, the lighter isotope was not considered of importance in nuclear reactions because of its low concentration. Now, to make a uranium bomb, it would be necessary to separate U-235 from U-238, which is a difficult task. Physical, not chemical, methods have to be used for the separation, since the two isotopes have identical chemical properties.
Complete Bibliography on Fission from the Alsos Digital Library for Nuclear Issues
Contributors
Frank A. Settle (Washington and Lee University)


