2: Discovery of the Neutron (1932)
- Page ID
- 19567
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The story begins in 1932, with the discovery of the neutron by Sir James Chadwick, an English physicist. Until 1932, the atom was known to consist of a positively charged nucleus surrounded by enough negatively charged electrons to make the atom electrically neutral. Most of the atom was empty space, with its mass concentrated in a tiny nucleus. The nucleus was thought to contain both protons and electrons because the proton (otherwise known as the hydrogen ion, H+) was the lightest known nucleus and because electrons were emitted by the nucleus in beta decay. In addition to the beta particles, certain radioactive nuclei emitted positively charged alpha particles and neutral gamma radiation. The symbols for these emissions are \(\beta^-\) or \(\ce{_{–1}e^0}\), \(\alpha^{2+}\) or \(\ce{_2^4He^{2+}}\), and \(_0^0\gamma\).


Sir James Chadwick. (Courtesy of the American Institute of Physics) (left), Lord Rutherford at Cambridge (right)
Twelve years earlier, Lord Ernest Rutherford, a pioneer in atomic structure, had postulated the existence of a neutral particle, with the approximate mass of a proton, that could result from the capture of an electron by a proton. This postulation stimulated a search for the particle. However, its electrical neutrality complicated the search because almost all experimental techniques of this period measured charged particles.
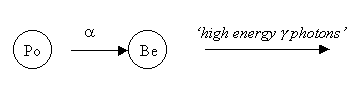
In 1928, a German physicist, Walter Bothe, and his student, Herbert Becker, took the initial step in the search. They bombarded beryllium with alpha particles emitted from polonium and found that it gave off a penetrating, electrically neutral radiation, which they interpreted to be high-energy gamma photons.

The Joliot-Curies in their laboratory. (Courtesy of the American Institute of Physics)
In 1932, Irene Joliot-Curie, one of Madame Curie’s daughters, and her husband, Frederic Joliot-Curie, decided to use their strong polonium alpha source to further investigate Bothe’s penetrating radiation. They found that this radiation ejected protons from a paraffin target. This discovery was amazing because photons have no mass. However, the Joliot-Curies interpreted the results as the action of photons on the hydrogen atoms in paraffin. They used the analogy of the Compton Effect, in which photons impinging on a metal surface eject electrons. The trouble was that the electron was 1,836 times lighter than the proton and, therefore, recoiled much more easily than the heavier proton after a collision with a \(\gamma\) photon. We now know that gamma photons do not have enough energy to eject protons from paraffin.
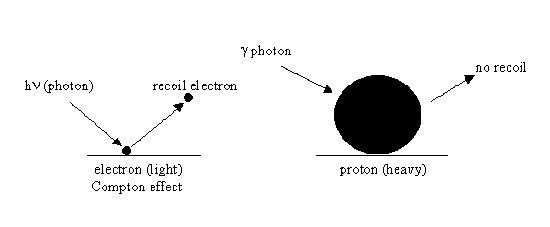
The Compton Effect
When James Chadwick reported to Lord Rutherford on the Joliot-Curies’ results, Lord Rutherford exclaimed, "I do not believe it!" Chadwick immediately repeated the experiments at the Cavendish Laboratory in Cambridge, England. He not only bombarded the hydrogen atoms in paraffin with the beryllium emissions, but also used helium, nitrogen, and other elements as targets. By comparing the energies of recoiling charged particles from different targets, he proved that the beryllium emissions contained a neutral component with a mass approximately equal to that of the proton. He called it the neutron in a paper published in the February 17, 1932, issue of Nature. In 1935, Sir James Chadwick received the Nobel Prize in physics for this work. You can read his lecture as he received his Nobel prize. It is interesting to note that the Joliot-Curies’ misinterpretation of their results cost them the Nobel Prize. (Not to worry; in 1935, they received the Nobel Prize in chemistry for their discovery of artificial radioactivity.)
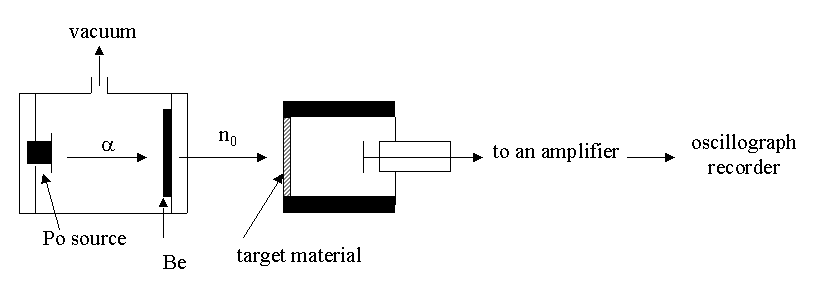
Chadwick's Apparatus
The search was over. Chadwick had found a new elementary particle, the third basic component of the nucleus. It increased the mass of elements without adding electrical charge. Two protons and 2 neutrons made a helium nucleus while 92 protons and 146 (or 143) neutrons made uranium, the heaviest known element. This not only changed our view of the nucleus, but also provided a new, relatively inexpensive means of probing the nucleus. Because the neutron was relatively massive but neutral, it was scarcely affected by the cloud of electrons surrounding the nucleus or by the positive electrical barrier of the nucleus itself; thus it could penetrate the nucleus of any element.
Consult Nature magazine for the original Letter to the Editor by James Chadwick regarding the "possible existence of a neutron," published February 27, 1932.
Complete Bibliography on Neutrons from the Alsos Digital Library for Nuclear Issues
Contributors
Frank A. Settle (Washington and Lee University)


