Intro to Solids
- Page ID
- 53634
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Relate the different types of solids to the 3 main types of bonding
- Describe some of the properties of solids
Solids are one of the most interesting and important topics in general chemistry because they are important for so many things. We use solids as building materials, to make tools of all kinds, to make computer chips, energy storage devices, solar cells, catalysts... Nearly all technology depends on the properties of solids.
Types of Solids
We can categorize solids in many ways. The most common way is by the type of bonding. There are three main types of bonding. So far we have focused on covalent bonding. There are two types of covalent solids: molecular and covalent-network. Molecular solids are made of covalent molecules, and the solids are held together by Van der Waals forces between the molecules. These molecular solids usually have low melting points and are easy to break, because the forces between molecules are weak. Covalent-network solids are different because there are no separate molecules: the whole solid is held together by covalent bonds between atoms. For example, in diamond, each C atom makes 4 covalent bonds to 4 other C atoms in a tetrahedral arrangement. Covalent-network solids usually have high melting points and are relatively hard because they are held together by very strong bonds. Diamond is the hardest material known, because C-C bonds are among the strongest single bonds known. (Could you make a covalent-network solid using the even-stronger bonds?)
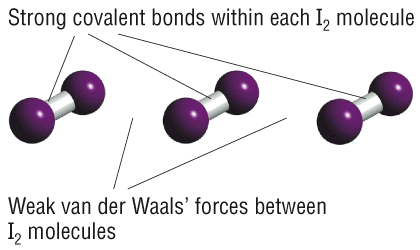

We have also talked a little bit about ionic bonding. Basically, you probably know that most ionic materials are solids at room temperature, and they are made of ions, such as a metal cation and a non-metal anion. Ionic solids are held together by strong electrostatic (Coulomb's law) forces between the ions, so they are usually hard and have high boiling points.
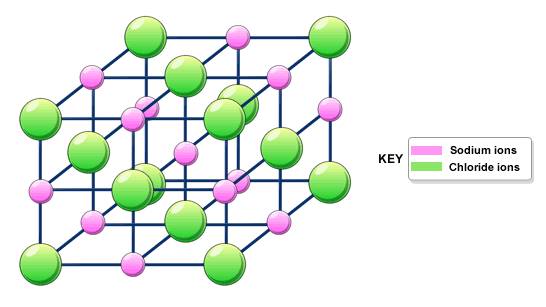
There are also many solids that are on the border between covalent and ionic: they are made of metals and non-metals, but both covalent bonding and ionic bonding are important. This includes most rocks, minerals and ceramics. For instance, sapphire, another of the hardest materials, is aluminum oxide.

Finally, there is metallic bonding. Metallic bonding is sort of like covalent bonding, because it involves sharing electrons. The simplest model of metallic bonding is the "sea of electrons" model, which imagines that the atoms sit in a sea of valence electrons that are delocalized (spread out) over all the atoms. Because there aren't specific bonds between individual atoms, metals are more flexible than covalent-network solids. The atoms can move around and the electron sea will keep holding them together. Some metals are very hard and have very high melting points, while others are soft and have low melting points. This depends roughly on the number of valence electrons that form the sea.
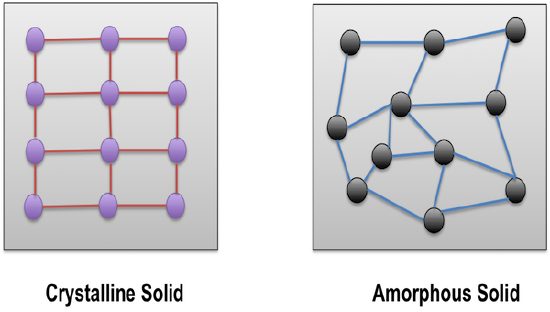
There are actually some other types of solids also. One way we can separate solids into categories is crystalline or amorphous. Crystalline solids have ordered arrangements of atoms or ions. Amorphous (which means "no-shape") solids have disorganized arrangements. Metals, ionic solids, covalent-network and molecular solids are usually at least sort of crystalline and orderly, although they are never completely perfect. Crystalline solids often have faces or facets (flat surfaces at particular angles), like most of the gem-stones used to make jewelry. An important type of amorphous materials are polymers, which include most plastics. Polymers are usually made of really long, skinny covalent molecules that are tangled together. They are often flexible. There are also amorphous stones, like opal (which is usually polished to a rounded shape, because it doesn't make nice facets).
We can also classify solids by their size. Nanomaterials are solids that have very small sizes. Nanomaterials can be made from metallic or covalent-network materials, but because of their small size, they have different properties.
Properties of Solids
As you read about solids, think about how the structure relates to the important properties of the solids that determine how we can use it. Here we will describe some of these properties.
For structural materials (materials that we use to build, support, protect) the strength and hardness can be very important. Hardness means how easy it is to change the shape of the material, such as by stretching or denting it, or how elastic it is in a collision. Strength of materials means how well they resist applied forces, such as compression or stretching. This is important if you want to build a bridge or a building, for example. We will describe solids as brittle if they break into pieces, such as ionic or covalent solids. Metals don't usually break into pieces, but they can stretch into wires (ductility) and be pounded into sheets (malleability).
Conductivity is another important type of property. Do the materials conduct electricity? If so, how much? Do they conduct sound or heat, and how well? We might also want to know about the reactivity of solids. Are they inert (non-reactive) at high temperature in acid? Do they catalyze (speed up) certain reactions on their surfaces? Do they absorb or emit light, and if so, at what energy? Are they magnetic? Are they porous (full of tiny holes)? All these properties can be important for different applications.
Valence and Solids
As you read about solids, it can be helpful to remember the ideas about valence we have discussed earlier. Different atoms have different numbers of valence electrons, which determines how many electrons they usually lose or gain, and how many bonds they usually make. Another way to think about this is with coordination number, which is how many other atoms one atom interacts with. In molecules, coordination numbers are usually pretty low, like 1-4. In ionic solids, each ion might have 2-8 neighbors. In metals, each atom might have 12 neighbors. The stable coordination number of an atom or ion depends on the situation, and how many electrons it needs. To form a covalent-network solid, at least some of the atoms in the formula have to make many bonds (like carbon or silicon, which usually makes 4 bonds). You can't make a good network with only atoms that form 2 bonds, because you can't have branches. In molecular solids, all the bonds are inside the molecule. In metals, in order to gain the electrons they want, because they start with fewer valence electrons, they usually have high coordination numbers, which requires that they have certain structures.
Defects
A really important topic in solids is defects or places where the arrangement of atoms isn't perfect. All examples of a particular molecule are exactly the same, because if one of the atoms is different or the arrangement is different, it would be a different molecule. But solids can be mostly one formula and structure, with some impurities or places where the structure isn't exactly orderly. In fact, solids always have defects. Many of the most important properties of solids depend on the defects, like having a few atoms of different types, or an atom missing, etc. So remember that sometimes the bulk description (how most of the solid is) isn't actually the most important part. The most important part might be the rare spot where a few atoms aren't in their places. This can make solids very confusing to study!
Outside Links
Contributors and Attributions
Emily V Eames (City College of San Francisco)

