Flame Tests
- Page ID
- 3673
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. For Group 1 compounds, flame tests are usually by far the easiest way of identifying which metal you have got. For other metals, there are usually other easy methods that are more reliable - but the flame test can give a useful hint as to where to look.
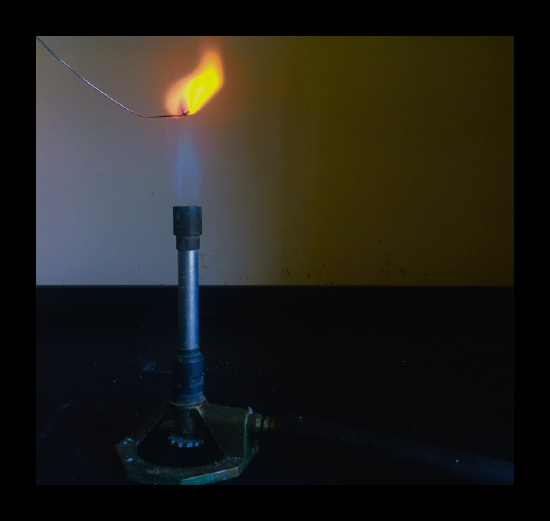
- Clean a platinum or nichrome (a nickel-chromium alloy) wire by dipping it into concentrated hydrochloric acid and then holding it in a hot Bunsen flame. Repeat this until the wire produces no color in the flame.
- When the wire is clean, moisten it again in the acid and then dip it into a small amount of the solid to be tested so that some sticks to the wire. Place the wire back in the flame.
- If the flame color is weak, it is often helpful to dip the wire back in the acid and put it back into the flame as if cleaning it. This should produce a very short but intense flash of color.
There will, in fact, always be a trace of orange in the flame if you use nichrome. Platinum is much better to use but is much, much more expensive. If you have a particularly dirty bit of nichrome wire, you can just chop the end off. You do not do that with platinum! Dilute hydrochloric acid can be used instead of concentrated acid for safety reasons, but does not always give such intense flame colors.
The colors in Table \(\PageIndex{1}\) are just a guide. Almost everybody sees and describes colors differently. I have, for example, used the word "red" several times to describe colors that can be quite different from each other. Other people use words like "carmine" or "crimson" or "scarlet", but not everyone knows the differences between these words - particularly if their first language is not English.
| Element | flame color |
|---|---|
| Lithium | red |
| Sodium | strong, persistent orange |
| Potassium | lilac (pink) |
| Rubidium | red (red-violet) |
| Cesium | blue/violet (see below) |
| Calcium | orange-red |
| Strontium | red |
| Barium | pale green |
| Copper | blue-green (often with white flashes) |
| Lead | gray-white |
What do you do if you have a red flame color for an unknown compound and do not know which of the various reds it is? Get samples of known lithium, strontium (etc) compounds and repeat the flame test, comparing the colors produced by one of the known compounds and the unknown compound side by side until you have a good match.
The Origin of Flame Colors
If you excite an atom or an ion by very strong heating, electrons can be promoted from their normal unexcited state into higher orbitals. As they fall back down to lower levels (either in one go or in several steps), energy is released as light. Each of these jumps involves a specific amount of energy being released as light energy, and each corresponds to a particular wavelength (or frequency). As a result of all these jumps, a spectrum of lines will be produced, some of which will be in the visible part of the spectrum. The color you see will be a combination of all these individual colors.
In the case of sodium (or other metal) ions, the jumps involve very high energies and these result in lines in the UV part of the spectrum which your eyes can't see. The jumps that you can see in flame tests come from electrons falling from a higher to a lower level in the metal atoms. So if, for example, you put sodium chloride which contains sodium ions, into a flame, where do the atoms come from? In the hot flame, some of the sodium ions regain their electrons to form neutral sodium atoms again. A sodium atom in an unexcited state has the structure 1s22s22p63s1, but within the flame there will be all sorts of excited states of the electrons. Sodium's familiar bright orange-yellow flame color results from promoted electrons falling back from the 3p1 level to their normal 3s1 level.
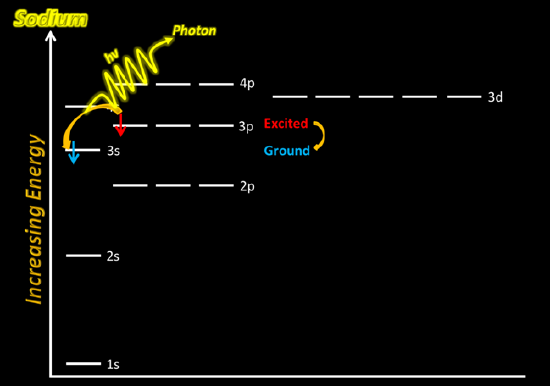
The exact sizes of the possible jumps in energy terms vary from one metal to another. That means that each different metal will have a different pattern of spectral lines, and so a different flame color. Flame colors are produced from the movement of the electrons in the metal ions present in the compounds. For example, a sodium ion in an unexcited state has the electron configuration 1s22s22p6. When heated, the electrons gain energy and can be excited into any of the empty higher-energy orbitals—7s, 6p, 4d, or any other, depending on the amount of energy a particular electron happens to absorb from the flame. Because the electron is now at a higher and more energetically unstable level, it falls back down to the original level, but not necessarily in one transition.
The electron transitions which produced lines in the visible spectrum involved atoms rather than ions.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)

