Clemmensen Reduction
- Page ID
- 5667
The reaction of aldehydes and ketones with zinc amalgam (Zn/Hg alloy) in concentrated hydrochloric acid, which reduces the aldehyde or ketone to a hydrocarbon, is called Clemmensen reduction.
Introduction
This alternative reduction involves heating a carbonyl compound with finely divided, amalgamated zinc in a hydroxylic solvent (often an aqueous mixture) containing a mineral acid such as HCl. The mercury alloyed with the zinc does not participate in the reaction, it serves only to provide a clean active metal surface.
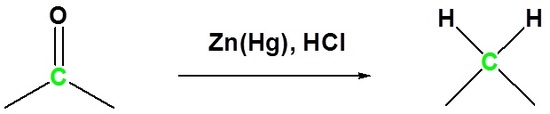
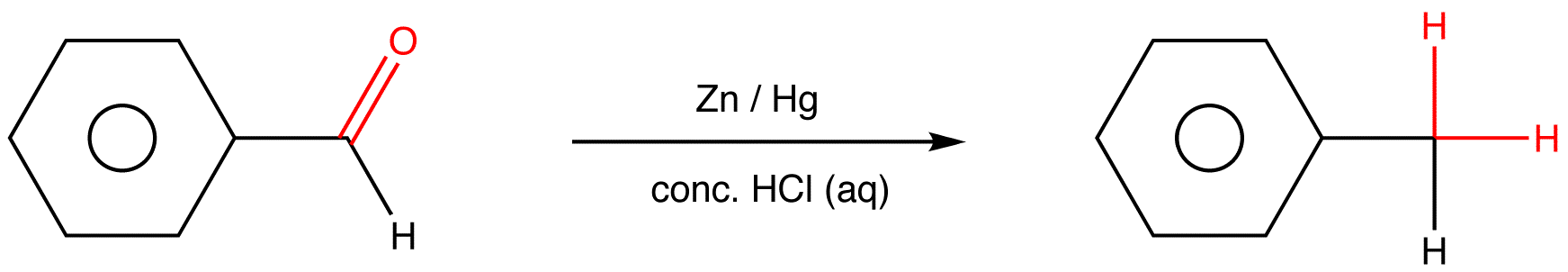
Example

Possible mechanism
The mechanism of Clemmensen reduction is not fully understood; intermediacy of radicals are implicated. Clemmensen reduction is complementary to Wolff-Kishner reduction, which also converts aldehydes and ketones to hydrocarbons, in that the former is carried out in strongly acidic conditions and the latter in strongly basic conditions.
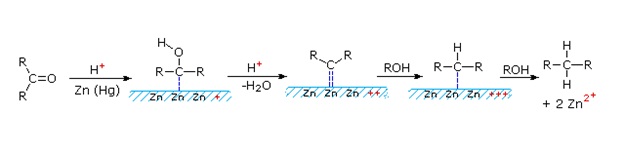
References
- Clemmensen, E. Ber. Dtsch. Chem. Ges. 1913, 46, 1837–1843.
- Clemmensen, E. Ber. Dtsch. Chem. Ges. 1914, 47, 51–63.
- Clemmensen, E. Ber. Dtsch. Chem. Ges. 1914, 47, 681–687.
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Gamini Gunawardena from the OChemPal site (Utah Valley University)

