2: Building Your Molecules - Energy Optimization
- Page ID
- 274071
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Before we proceed into building molecules, I would like to first briefly introduce Energy Optimization or sometimes known as Energy Minimization. Avogadro finds the most stable form, lowest energy, of the molecule by minimizing all the interactions within the molecule. It does so by using advanced algorithms by applying a force field.
1) We are going to begin building Methane (CH4). Click the pen, or draw tool, on the tool ribbon as shown in Figure \(\PageIndex{1}\) below.
2) The display types window and tool settings window should be on your screen. If not, simply click on the tool settings and display settings next to the tool ribbon. From the draw settings window, you are able to select the elements you wish to draw with. There is also an adjust hydrogens checkbox. This adds hydrogens to the atom based off the valency of the atom in order to fulfill the octet rule. For example, click on element drop box in the draw settings window and select carbon, see figure II. On the black screen, click on the spot you wish to put the atom. The carbon comes up with four hydrogen atoms (CH4), see figure II. If you do this with oxygen, the atom will appear with two hydrogens (try some other atoms on your own and see the octet rule in action).
Note: The actually setting that would be found in the tool setting window depends on which tool you have selected and are working with.
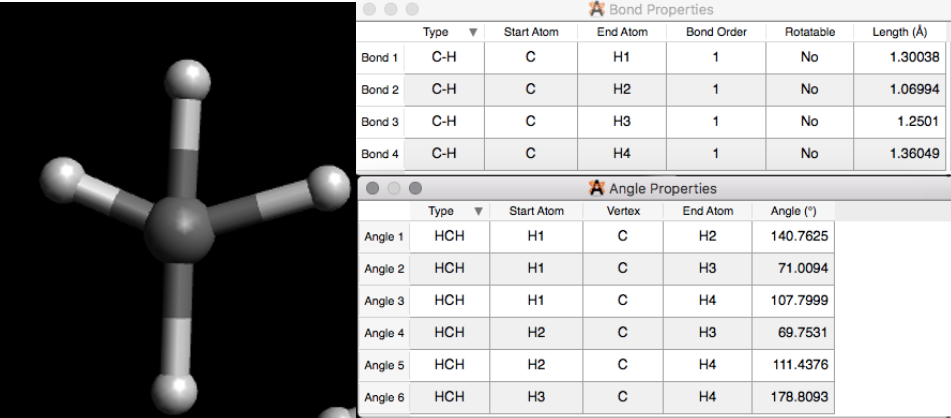 Figure \(\PageIndex{2}\): To draw settings with carbon and adjust hydrogens selected (left). Drawn Methane, CH4 (right) (Copyright; author via source)
Figure \(\PageIndex{2}\): To draw settings with carbon and adjust hydrogens selected (left). Drawn Methane, CH4 (right) (Copyright; author via source)3) CH4 (methane) has a tetrahedral geometry. The angle and bond distances are as follows:
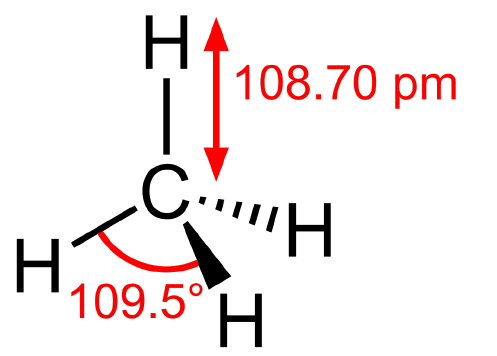
Figure \(\PageIndex{3}\): 3-D Diagram of Methane (Copyright; author via source)
where the distance is given in picometers. Please note Avogadro gives the distance in angstroms. To check the angles, go to angle properties (see step 5 in Section 1). To check the lengths, go to bond properties (see step 5 in Section 1). Because methane is a simple molecule, and if you have the adjust hydrogen checked off, then Avogadro usually gives the correct geometry. See below, figure IV, for the bond and angle properties of the methane drawn in Figure \(\PageIndex{2}\). Geometry is important in energy optimization.

4) For the purpose of understanding energy optimization, let’s draw methane without the adjust hydrogens. To do so, click the draw tool, select carbon from the element list, and place anywhere on the black screen. Then select hydrogen. In order to make the bond between atoms, you must CLICK AND HOLD on the starting atom (carbon), and then DRAG from the clicked atom (carbon) over to the position in which you want the other atom (hydrogen) to be. See figure VI for the drawn molecule.
NOTE: If you wish to make a single bond to a double bond, click on the bond once. To make it a triple bond, click on it once more.
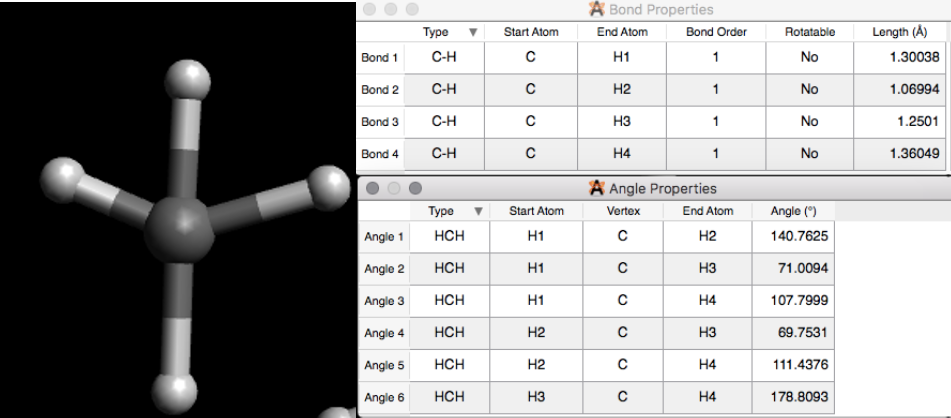 Figure \(\PageIndex{5}\): Methane drawn without the adjust hydrogens setting (left). Properties of the Methane(Right).(Copyright; author via source)
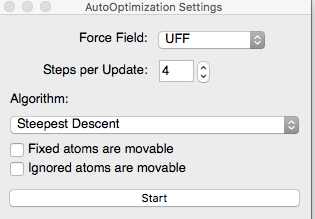
Figure \(\PageIndex{5}\): Methane drawn without the adjust hydrogens setting (left). Properties of the Methane(Right).(Copyright; author via source)The length and angles are not in the correct geometry. To fix this, we are going to use the auto optimization tool, Figure \(\PageIndex{6}\). Avogadro applies a force field (using some very fancy algorithms of course), to obtain the optimized structure. Working with larger and more complex structures could sometimes lead to using different optimization options. But, for now we will use the settings shown in Figure \(\PageIndex{6}\) for the simple and small molecules we are working with. Click on Start. You Stop the optimization once the change in energy (dE) is equal to zero or about zero, Figure \(\PageIndex{6}\). The E is equal to the lowest energy value. Check the properties. You should now have the correct geometry.

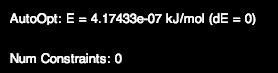
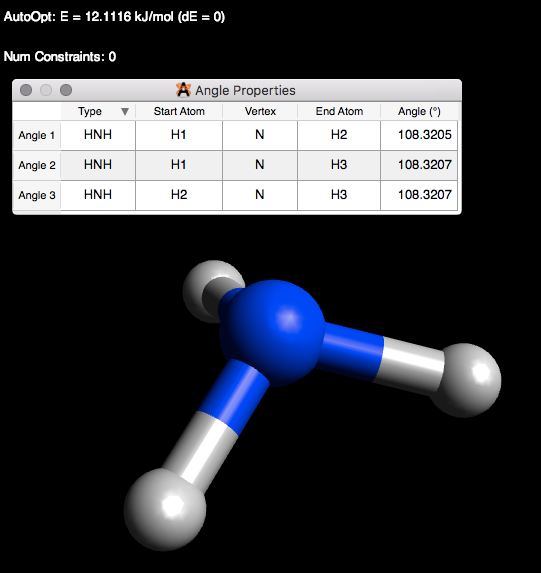
5) Now let’s do Ammonia (NH3). Ammonia has a trigonal geometry. The lone pair of electrons repel the hydrogen atoms making an angle of 107°. Draw ammonia with the adjusted hydrogens. Check the bond angles. If the bond angles do not equal 107°, then optimize the structure. Sometimes, you may not get the exact bond angle using the auto optimization tool. This is usually not a problem if the angle is almost or about the desired angle. However, you could also get a better geometry through one more step. Go to the toolbar under Extension > Optimize Geometry. Check to see if you now have an angle closer to the one you desire. See Figure \(\PageIndex{7}\) for drawn ammonia and the angle properties.
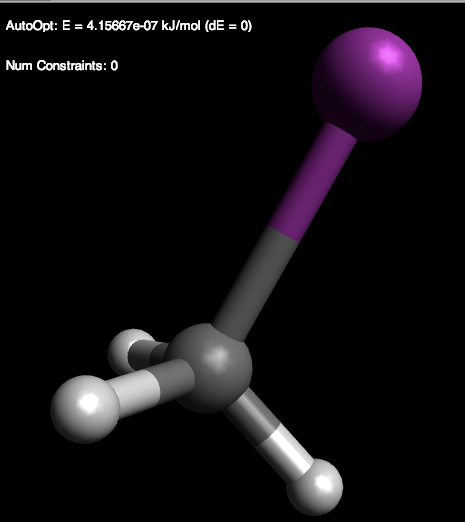
6) Now, we will look at an optimized iodomethane (CH3I). See Figure \(\PageIndex{8}\). Try optimizing the structure on your own!
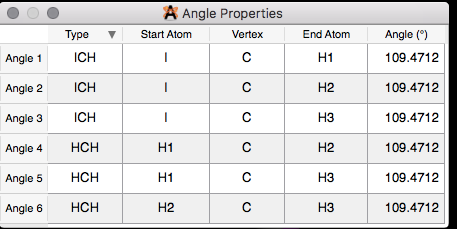
Try drawing this structure for fun and practice!
The benzene ring (C6H6) is very important organic compound and is classed as an aromatic hydrocarbon. Fun fact: the word “aromatic” comes from the fact that these compounds usually produces a sweet aroma
Figure \(\PageIndex{1}\): Copy and Paste Caption here. (Copyright; author via source)

