Carbonic Anhydrase 2
- Page ID
- 2479
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)An anhydrase is (defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this "reverse" reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid. It is important to realize that enzymes are such a key factor in chemistry because without a catalyst, a lot of the reactions would occur so slow that it would never even happen. The presence of a catalyst, in this case carbonic anhydrase, shows the evolution and adaptation of humans and plants as a whole. This can be observed from the following chemical reaction which is also reversible:
\[HCO_3^- + H^+ \rightarrow H_2CO_3 \rightarrow CO_2 +H_2O \tag{1}\]
The most studied derivatives of carbonic anhydrases, which are focused on in this article, are α-carbonic anhydrase and β-carbonic anhydrase which occurs in animals and plants. Without the presence of the carbonic anhydrase catalyst a lot of reactions in an organism would never even occur. The enzyme interconverts between carbonic acid and the production of bicarbonate and protons under the following chemical reaction:
\[CO_2 + H-2O \rightarrow \text{carbonic anhydrase} \rightarrow HCO_3^- + H^+ \tag{2}\]
with a pka is around 6.3. Therefore at neutral pH=7, it favors the production of \(HCO_3^-\) which is important in the blood for regulation of concentrated \(CO_2\) in the body.
Structure
Carbonic anhydrase is part of family of metalloenzymes which contain a metal atom in its active site. The metal atom usually associated with derivatives of carbonic anhydrases is the zinc atom.
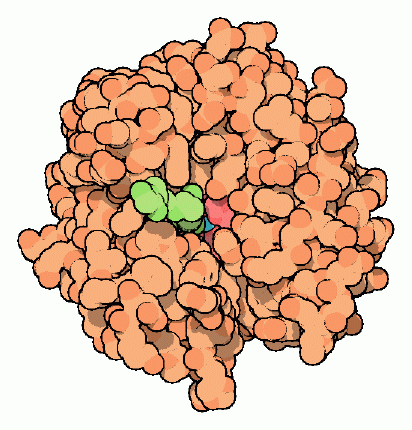
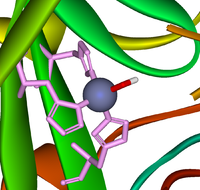
Figure 1: Nitrogen in active site (purple), Histidine 94,96 and 119 in pink and the final coordination is usually water or a hydroxyl ion. 3
The zinc atom has a coordination number of four as it is coordinated by three imidazole nitrogen atoms from three different histidines 94, 96, 119. The final coordination site is occupied by water or a hydroxyl ion.Because of the three histidines and one hydroxyl molecule, the coordination structure surrounding the zinc atom is almost completely tetrahedral.4
Symmetry
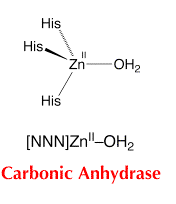
Figure 2: structure of carbonic anhydrase. 7
By assuming that the zinc cation is almost tetrahedral, we can predict it to have a point group of C3v. It is not a linear molecule because it is of tetrahedral shape. The final coordination of the hydroxyl group connected to the zinc atom makes the ligand structure not Td. There is definitely a principal access running down the zinc atom connected with the hydroxyl ion which is a C3. You can imagine it running down the Zn-O bond. There is not a σh due to the hydroxyl group. The ligand group at the active site does contain 3 σv’s along each of the His-Zn-O bond. Professors from Yale University were able to show that carbonic anhydrase was IR active by inhibiting the enzyme with an azide anion.5 This can be observed by the following table of \(C_{3v}\):
| C3v | E | 2C3 | 3σv | ||
| A1 | 1 | 1 | 1 | z | x2+y2, z2 |
| A2 | 1 | 1 | -1 | Rz | |
| E | 2 | -1 | 0 | (Rx, Ry), (x,y) | (xz, yz) (x2-y2, xy) |
- Na1: 1/6 (1*1*3 +0 + 3*1*1)= A1
- Na2: 1/6(1*1*3+ 0+ -3) = 0
- Na3: 1/6(1*2*3+0+0)= E
- Γr: A1 + E
IR active bands appear in the \(E\) column as either \(x\),\(y\) and \(E\) is in our reducible representation. Raman bands appear in any kind of multiplication function, for example x2-y2 ,which appears in both the A1 row and the E row. \(A_1\) should be Raman active and \(E\) should be both IR and Raman active for alpha carbonic anhydrase which is confirmed.
Reactions
The zinc atom is highlighted gray in figure 2 surrounded by the the three histidine molecules highlighted in yellow. There are even more histidines (His64, 106, 199). This causes the O-H bond to be a weak bond and as a fourth histidine is placed close to the water molecule, the histidine accepts a proton leaving a hydroxyl ion.2 This is stabilized due to the positive charge on the zinc atom. It is this negative charge on the hydroxyl ion that later undergoes a nucleophilic attack on the CO2 molecule producing a bicarbonate anion and protons. The figure on the top right shows the intermediates of the hydroxyl anion bound first to the zinc atom and then on the bottom right it shows the hydroxyl making a bicarbonate ion.
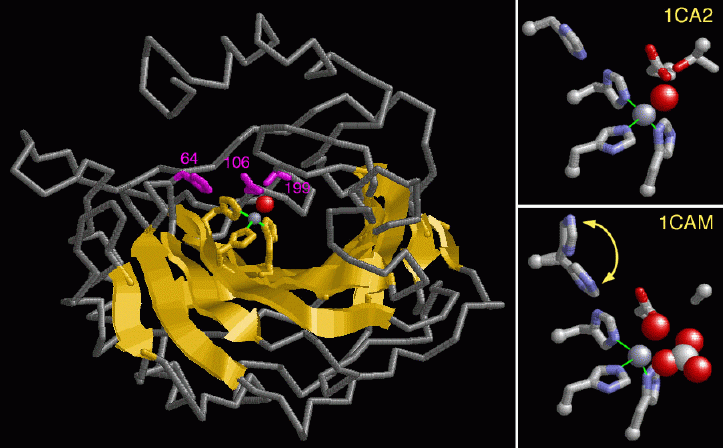
Figure 3: Taken from RCSB-PDB entry ICA-2 (http://www.rcsb.org/) 6
Functions
Carbonic anhydrase is seen in the blood. It first catalyzes the conversion of CO2 to bicarbonate and carbonic acid in red blood cells. After arriving to the lungs, this same enzyme reconverts bicarbonate ions back to CO2 which we then breathe out.6 Carbonic anhydrase is incredibly important in the body. It can regulate the CO2 concentration which by correlation regulates the pH of a body. In our stomach lining it plays a role in secreting acid, while the same enzyme helps to make pancreatic juices alkaline and our saliva neutral.6. This enzyme is found everywhere in the body. It is found in saliva, in kidneys to regulation pH and in cells which regulate both water and acid concentrations. Currently, there are researches trying to use carbonic anhydrase to treat glaucoma in the eyes by breaking down molecules in the fluid that causes pressure in the eye which damages it.
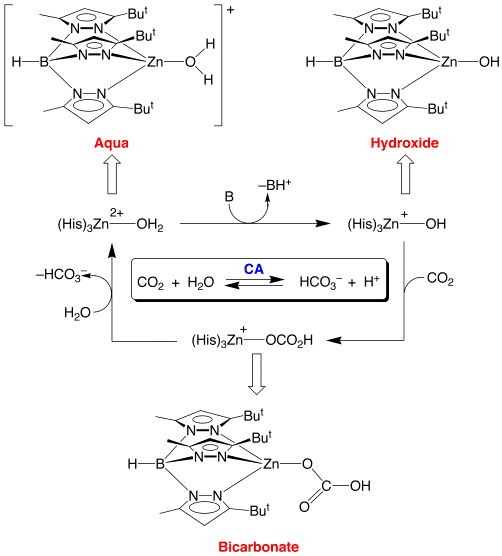
Figure 4 showing the importance of converting CO2 in the body. 7
In plants a form of β-carbonic anhydrase is found. Plants store CO2 as bicarbonate ions which carbonic anhydrase helps fix and then release. In summary, carbonic anhydrase plays a role in converting bicarbonate ions back to carbon dioxide for photosynthesis.6
References
- http://en.wikipedia.org/wiki/Carbonic_anhydrase
- http://www.rcsb.org/pdb/explore/explore.do?structureId=1CA2
- http://en.wikipedia.org/wiki/Metalloprotein#Carbonic_anhydrase
- Riepe, Mary E., and Jui H. Wang. "Infared Studies on the Mechanism of Action of Carbonic Anhydrase." The Journal of Biological Chemistry (1968): 2779-787. Web. 24 May 2010.
- "RCSB PDB : Structure Summary for 1CA2 - REFINED STRUCTURE OF HUMAN CARBONIC ANHYDRASE II AT 2.0 ANGSTROMS RESOLUTION." Web. 25 May 2010. <http://www.rcsb.org/pdb/explore/expl...ructureId=1CA2>.
- "Graduate Committee." Columbia University in the City of New York. Web. 25 May 2010. <http://www.columbia.edu/cu/chemistry...rkin/zinc.html>.

