Carbonic Anhydrase 1
- Page ID
- 2478
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)I. Introduction
Carbonic anhydrase (CA) are widespread metalloenzymes in higher vertebrates, wherein these zinc enzymes play crucial physiological roles.
1. The primary function:
Interconvert CO2 and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
2. There are at least 14 different α -carbonic anhydrase isoforms:
➔Some of these isozymes are,
- Cytosolic: CA I, CAII, CAIII, and CAVII
- Membrane bound: CA IV, CA IX, CA XII and CA XIV
- Mitochondrial: CA V
- Secrected in the saliva: CA VI
➔Three known acatalytic forms,
- Carbonic anhydrase- related proteins (CARPs) VIII
- CARPs X
- CARPs XI
3. CA are omnipresent metalloenzymes present in prokaryotes and eukaryotes and encoded by three distinct evolutionarily unrelated gene families:
· α -CAs: in vertebrates, bacteria, algae, and cytoplasm of green plants.
· β -CAs: predominantly in bacteria, algae and chloroplasts of both monocotyledons and dicotyledons.
· γ -CAs: mainly in archaea and some bacteria.
Representatives of the β - and γ -CA family: highly abundant in plants, bacteria and archaea.
Representatives of the α-CAs family possesses a high versatility.
· δ-CAs and ε-CAs are the two new families have been discovered.
4. Basic chemical property:
· β -CA and γ -CA family catalyzes of the reversible hydration of carbon dioxide to form bicarbonate anion and proton; α-CA is able to catalyze other hydrolytic processes.
II. Example CA:Carbonic anhydrase [88]
A. The structure of the carbonic anhydrase [88]
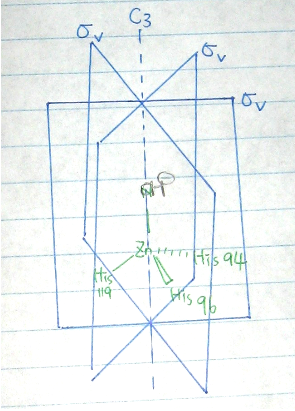
Point group:This molecule is not the linear; it does not have Td, Oh or Ih symmetry; there is a principal Cn (C1) axis; there are no nC2 axes perpendicular to the Cn axis, there is no σh plane perpendicular to the Cn axis; and there are no nσv planes containing the Cn axis ➭ Therefore, this molecule is C1, and no IR or Raman data is expected.
Symmetry Element:E
B. The structure of the metal active sites in the enzyme
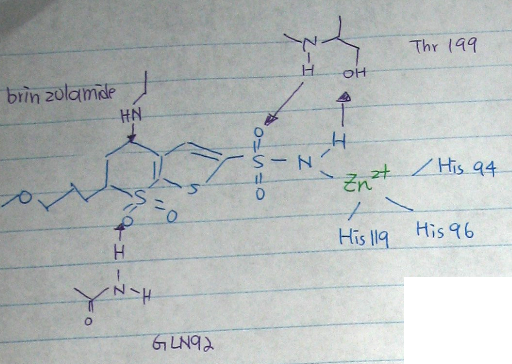
- [Fig.1] active site: The zinc (II) is coordinated by the rings of three His (94, 96, and 119) and a water molecule/ hydroxide ion. This causes polarization of the hydrogen-oxygen bond, making the oxygen slightly more negative, in order to weaken the bond.
- The reaction of the enzyme reactions
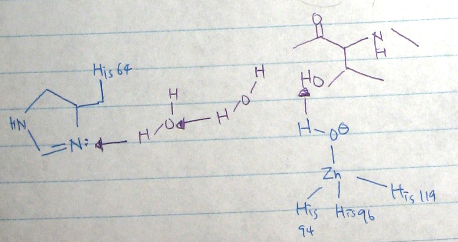
↑ [Fig.2] active site of the reaction of the carbonic anhydrase [88]
- Point group:
This molecule is not the linear; it does not have Td, Oh or Ih symmetry; there is a principal Cn (C3) axis; there are no nC2 axes perpendicular to the Cn axis, there is no σh plane perpendicular to the Cn axis; and there are nσv (3σv)planes containing the Cn axis => Therefore, this molecule is C3v.
- Symmetry Elements
E, C3, 3σv(see Figure 1.)
C. Determine the IR and Raman spectra
| C3V | E | 2C3 | 3σv |
|
|
| A1 | 1 | 1 | 1 | z | z2+y2, z2 |
| A2 | 1 | 1 | -1 | Rz |
|
| E | 2 | -1 | 0 | (x,y) (Rx,Ry) | (x2-y2, xy) (xz, yz) |
| ΓHis | 3 | 0 | 1 |
nA1= 1/6 [1.1.3+2.1.0+3.1.1]=1A1
nA2= 1/6 [1.1.3+2.1.0+3.-1.1]= 0
nE= 1/6 [1.2.3+2.-1.0+3.0.1]= 1E
Conclusion: ΓHis = 1A1 + 1E
- IR: Γvib must contain one of the x,y,z fuctions to be observable.
=> 1A1 + 1E
- Raman: Γvib must contain one of the x2, y2, z2, xy, xz, yx or combinations such as x2+y2, x2-y2, 2z2-x2-y2 fuctions to be observable.
=> 1A1 + 1E
Conclusion:2 bands in both spectra.
E. Active site's immediate ligand environment
The geometry of the active site is Tetrahedral. In tetrahedral geometry the overlap of metal d orbitals with the ligand orbitals is weak due to the geometry; metal d-orbitals point between the m-L bonds. In addition, the energy separation between the e- and 2t2 MO's is relatively small. Furthermore, metal complexes with tetrahedral geometry are always 'high spin'.
III. Related with human:
a) Carbonic anhydrase II
Human carbonic anhydrase II is one of the most efficient one in carbonic anhydrase isozymes, which catalyzesthe reversible hydration/dehydration of CO2 and water.
CO2 + H2O↔ HCO3- + H+
- It is widely found in human’s kidney, brain, Pancreas, gastric mucosa, skeletal muscle, retina and lens and other organizations organs.
- It involves in many human pathological and physiological processes, such as human acid-base balance, cancer, and osteoporosis glaucoma.
- Moreover, CA II has been an important drug target with more attention.
- Carbonic anhydrase II is one of the highest catalytic efficiency enzymes. In the absence of enzyme-catalyzed conditions, CO2 can also produce HCO3 and H+with water; but now the reaction rate is only 5 ×10- 2 s- 1. Furthermore, the reaction rate of carbonic anhydrase is one of the fastest of all enzymes, and its rate is typically limited by the diffusion rate of its substrates. Therefore, if the mechanism is under the enzyme-catalyzed conditions, the reaction rate will increase to 1. 6×106 s- 1.
b) Carbonic anhydrase Ⅸ
- Cancer: CA Ⅸis mainly found in cancer cells, but it does not exist in normal cells.
c) Carbonic Anhydrase inhibitors
- Carbonic anhydrase inhibitors: a class of pharmaceuticals that restrain the activity of carbonic anhydrase. Moreover, up to now, many inhibitors were discovered including some clinical therapeutic drugs.
- Types of inhibitors and its function:
| Name | | Function/cure |
| Acetazolamide | act as a mild diuretic | glaucoma, epilepsy (rarely), altitude sickness, and benign intracranial hypertension |
| Methazolamide | longer elimination half-life than acetazolamide | glaucoma (openangle) |
| Topiramate | weak inhibitor, subtypes II and IV (especially) | epilepsy and Lennox-Gastaut syndrome, and migrane headaches |
| Dorzolamide (sulfonamide) | carbonic anhydrase II inhibitor (mainly) | ocular hypertension or open-angle glaucom |
References
- Andrea, Claudiu, and Janet Conway. Carbonic Anhydrase. New york: Washington, D.C., 2004.
- Carbonic Anhydrase Inhibitors. Wikipedia. May 24, 2010 from <http://en.wikipedia.org/wiki/Carboni...ase_inhibitors>
- David and Richard E. Tashian. Biology and Chemistry of the Carbonic Anhydrases. New york: Washington, D.C., 1984.

