Chapter 1 Solutions
- Page ID
- 1116
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In-chapter Exercises
E1.1: Mass numbers are:
- 31P
- 32P
- 37Cl
E1.2:
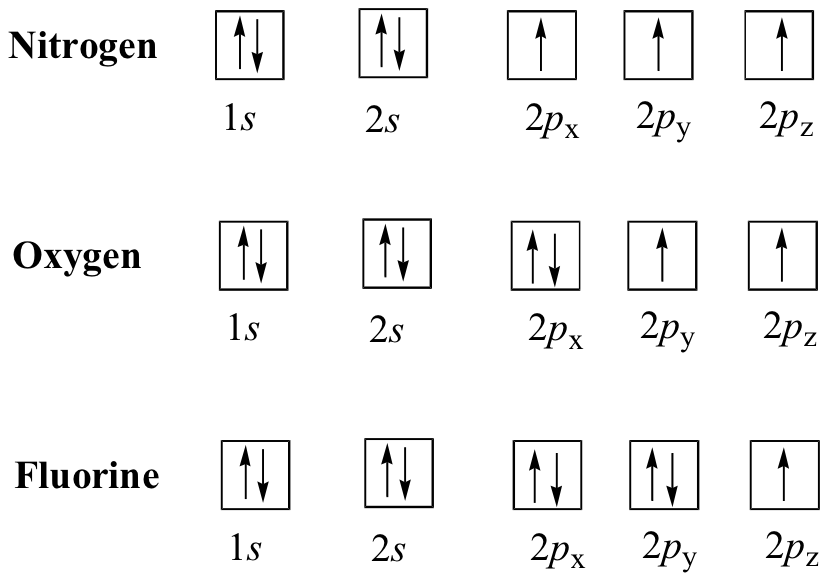
E1.3:
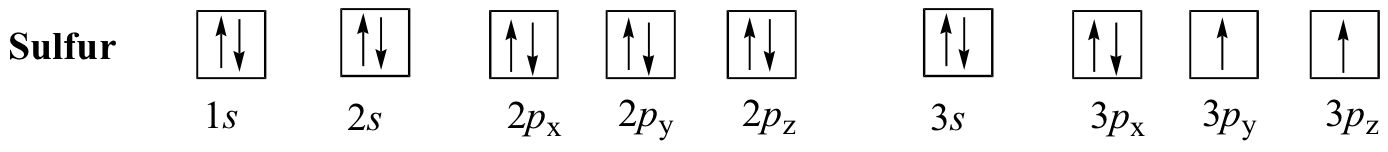
E1.4:
- a neutral magnesium atom: 1s22s22p63s2
- a Mg2+ atom: 1s22s22p6
- a neutral potassium atom: 1s22s22p63s23p64s1
- a K+ ion: 1s22s22p63s23p6
- a Cl- ion: 1s22s22p63s23p6
E1.5:
E1.6:
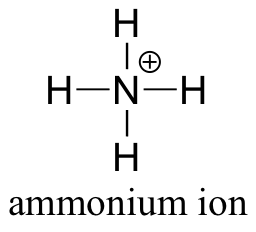
E1.7:
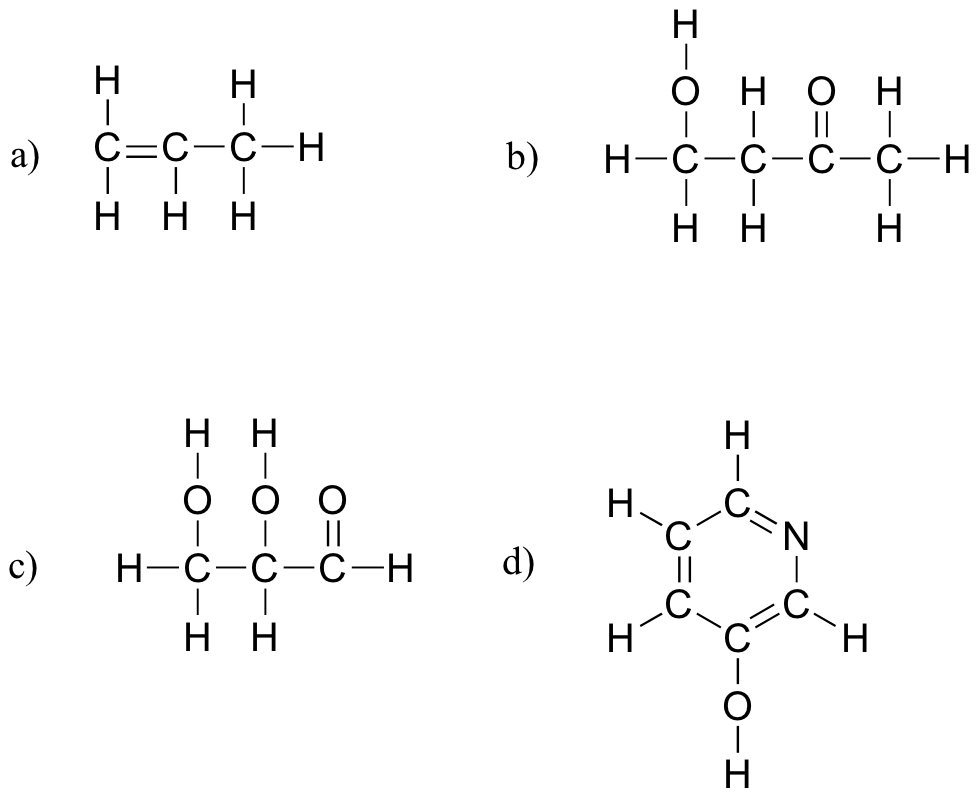
E1.8:
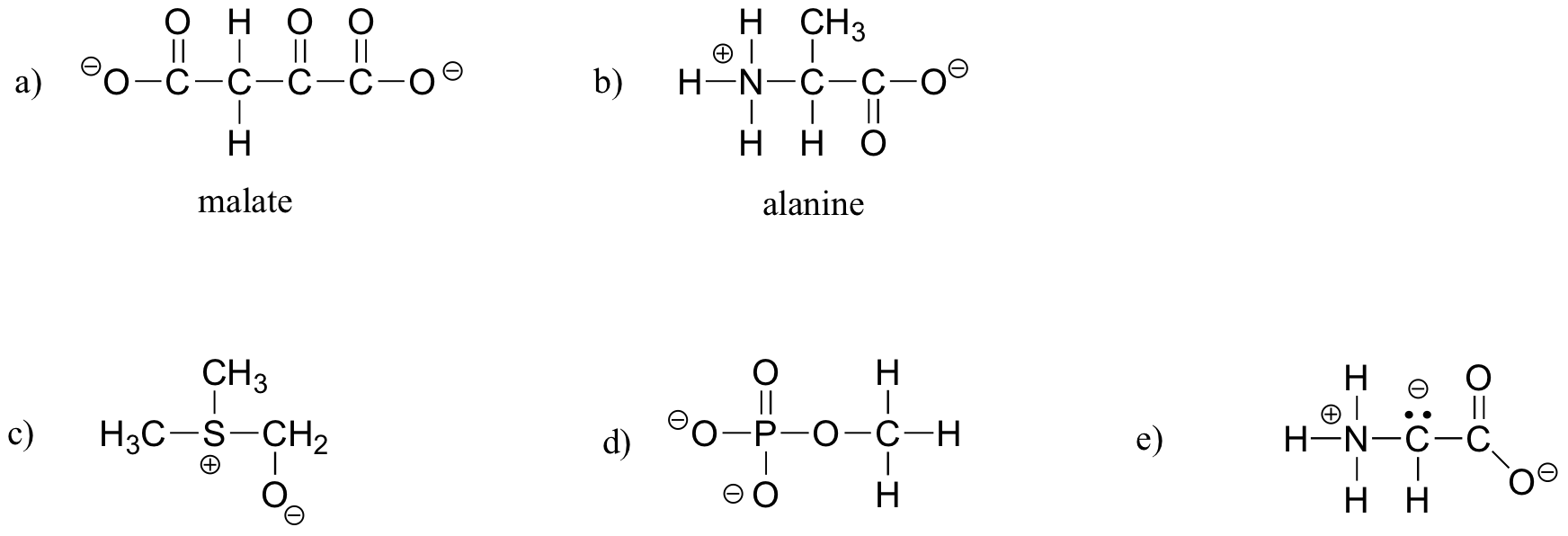
E1.9:
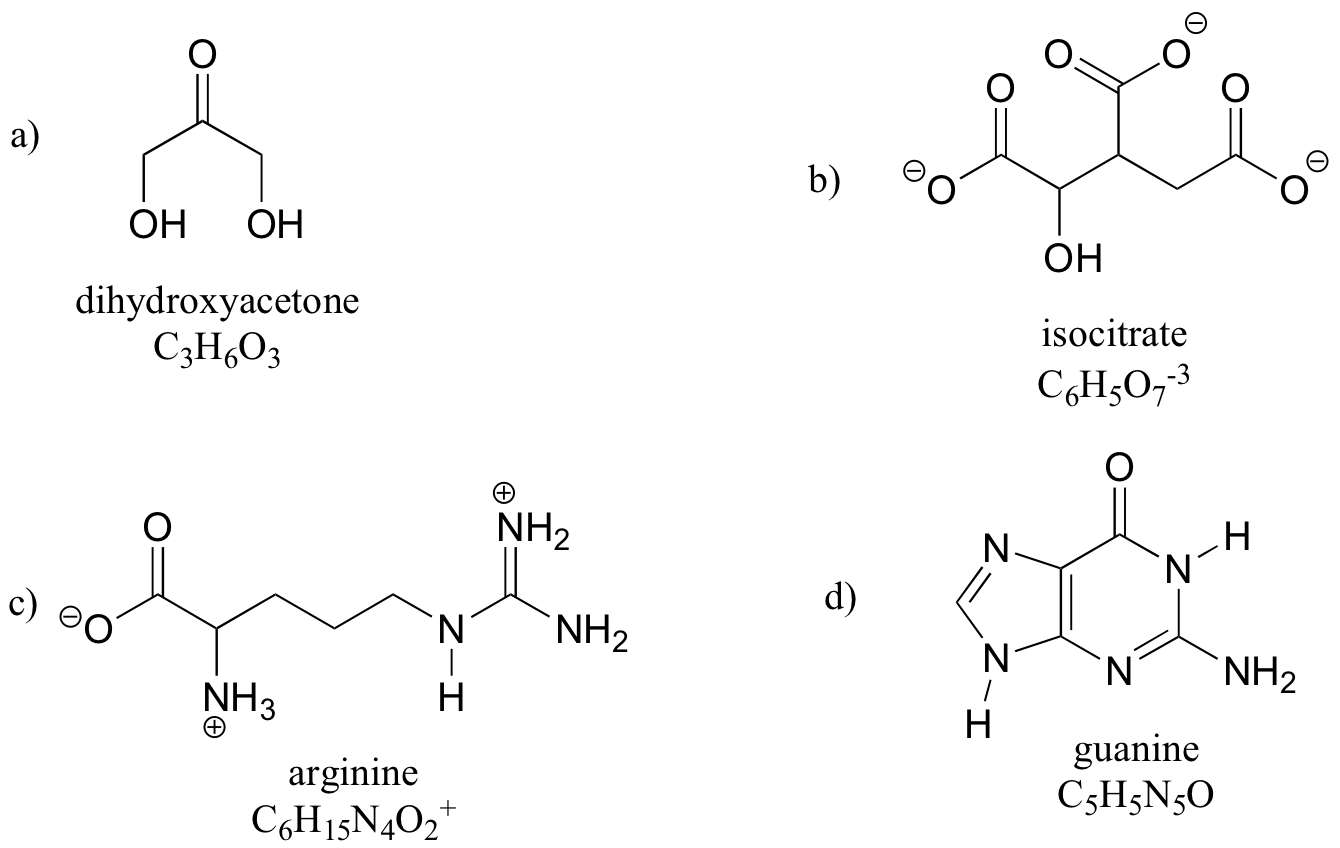
E1.10:
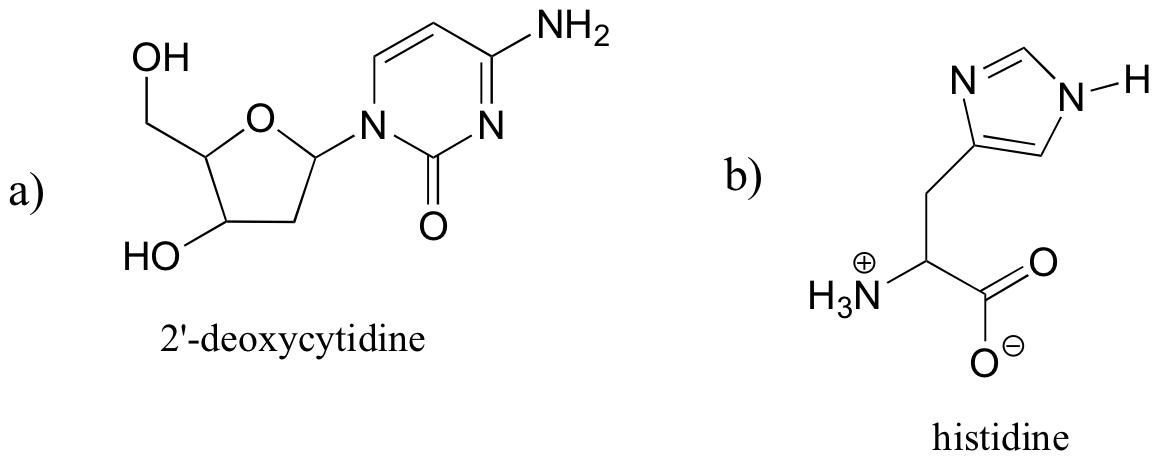
E1.11:
a) C5H12
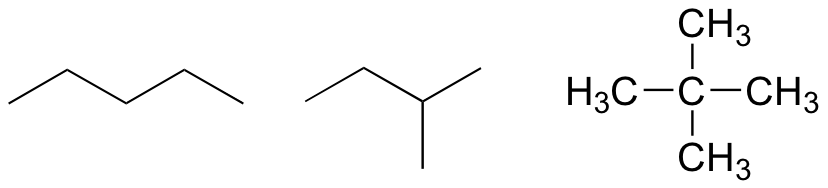
b) C4H10O
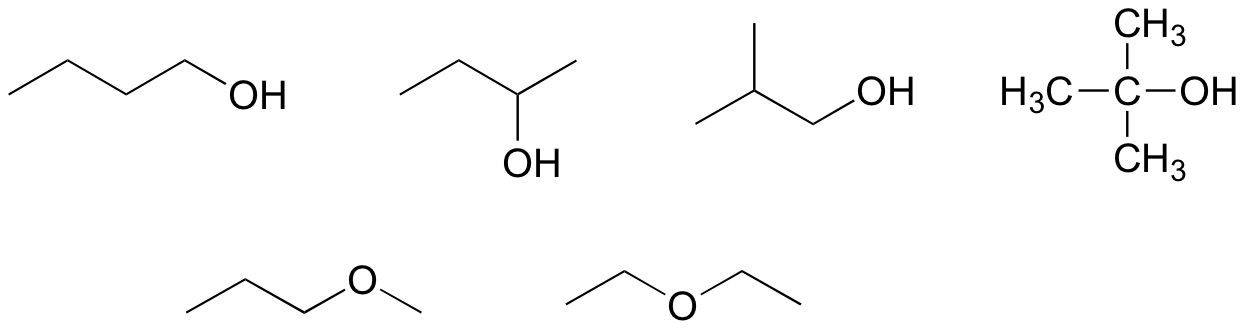
c) C3H9N
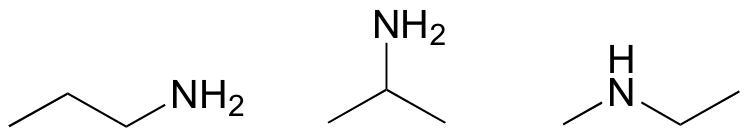
E1.12:
- a) 2-deoxycytidine: IHD = 5 (2 rings, 3 double bonds)
- b) histidine: IHD = 4 (2 rings, 2 double bonds)
- c) C9H10NOI: IHD = 5
- d) an ion with the molecular formula C6H11O7- : IHD = 1
E1.13:
- a) carboxylate, sulfide, aromatic, two amide groups (one of which is cyclic)
- b) tertiary alcohol, thioester
- c) carboxylate, ketone
- d) ether, primary amine, alkene
E1.14:
The following molecules (and many others) fit the descriptions in the problem:
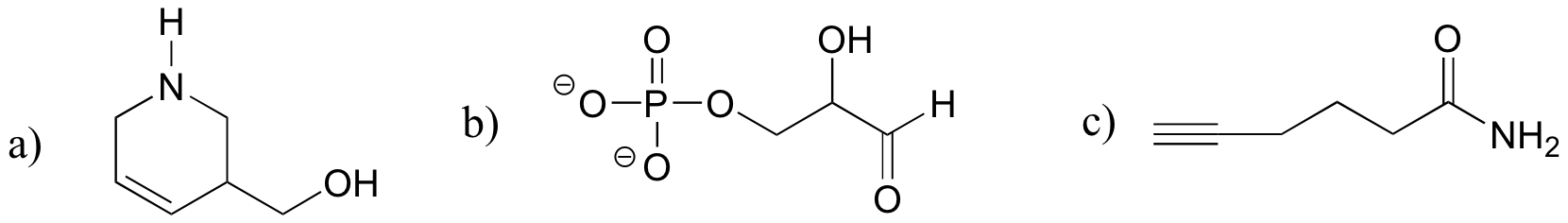
E1.15:
- acetic acid: ethanoic acid
- chloroform: trichloromethane
- acetone: 2-propanone
E1.16:
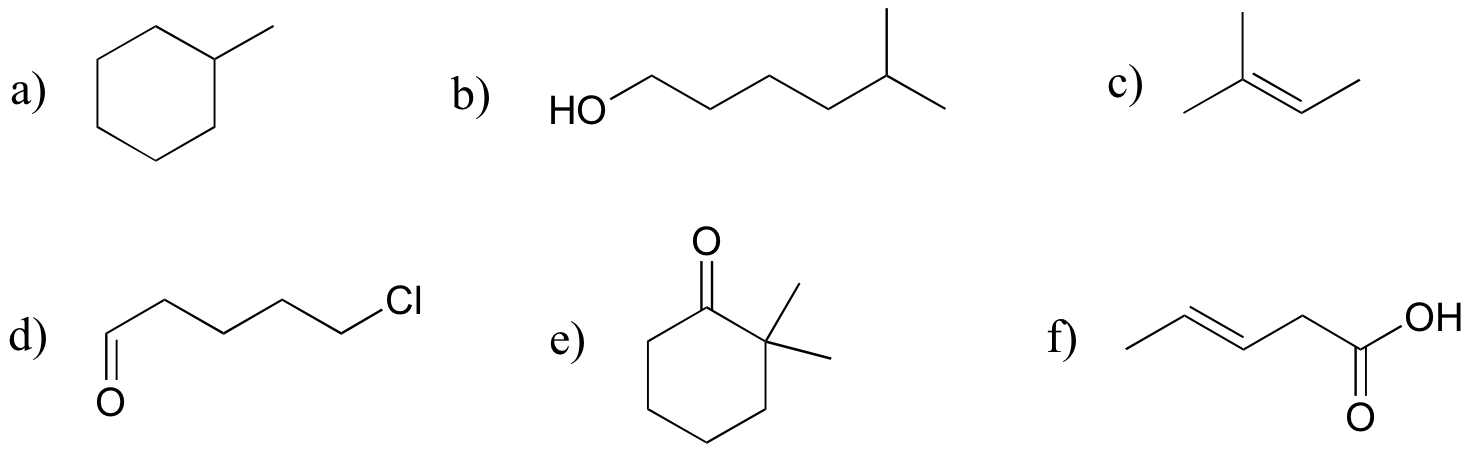
E1.17:
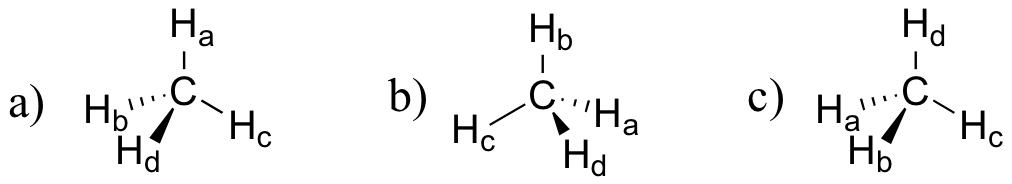
E1.18:
The bonding in chloroform consists of three of the sp3 hybrid orbitals on the central carbon atom overlapping with 3p orbitals on clorine. The fourth bond is an overlap between the fourth sp3 orbital on carbon and the 1s orbital of hydrogen.
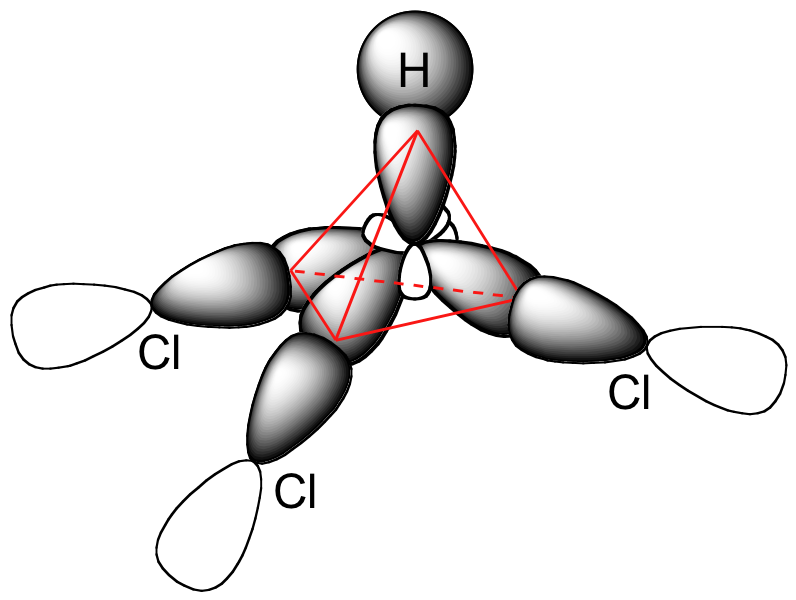
E1.19:
Both the carbon and the nitrogen atom in CH3NH2 are sp3-hybridized. The C-N sbond is an overlap between two sp3 orbitals.
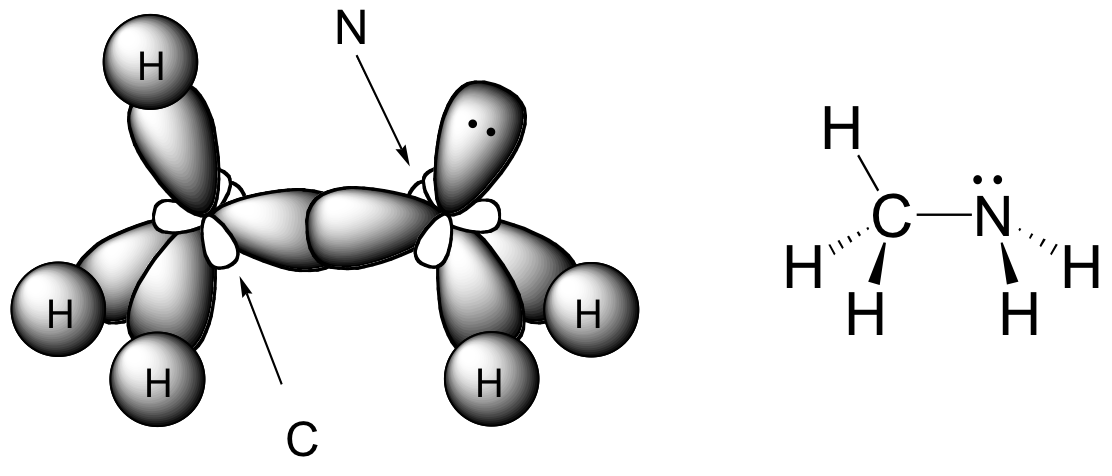
E1.20:
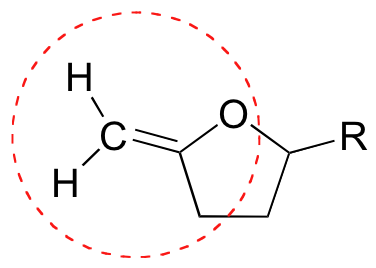
E1.21:
- a) Csp2-Csp2
- b) Csp2-Csp3
- c) Csp2-Nsp2
- d) Csp3-Nsp3
E1.22:
The three bonds that are drawn as solid wedges should be drawn as lines – they are all coming out of planar, sp2-hybridized carbons and should be shown lying in the plane of the page.
E1.23:
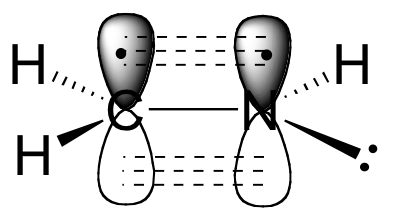
The carbon and nitrogen atoms are bond sp2-hybridized. The carbon-nitrogen double bond is composed of a s bond formed from two sp2 orbitals, and a pi bond formed from the side-by-side overlap of two unhybridized 2p orbitals.
E1.24:
- The sigma bond indicated by this arrow is formed by the overlap of an sp2 orbital of a carbon atom and an sp3 orbital of a carbon atom.
- The sigma bond indicated by this arrow is formed by the overlap of an sp2 orbital of a carbon atom and an sp2 orbital of a carbon atom.
- The sigma bond indicated by this arrow is formed by the overlap of an sp2 orbital of a nitrogen atom and an s orbital of a hydrogen atom.
- The sigma bond indicated by this arrow is formed by the overlap of an sp2 orbital of a carbon atom and an sp3 orbital of a carbon atom.
- The sigma bond indicated by this arrow is formed by the overlap of an sp3 orbital of a carbon atom and an sp3 orbital of a carbon atom.
End-of-chapter problems
P1.1:
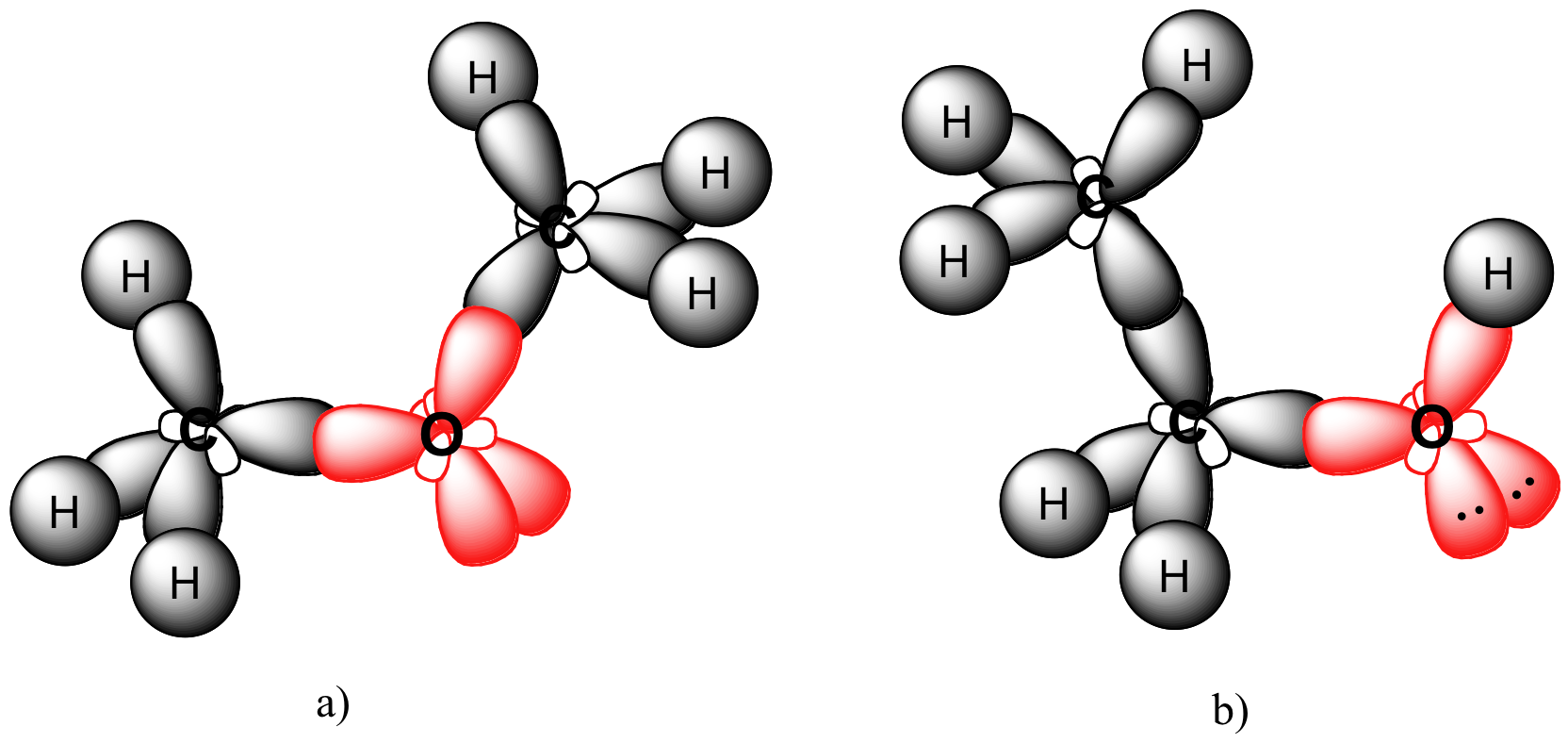
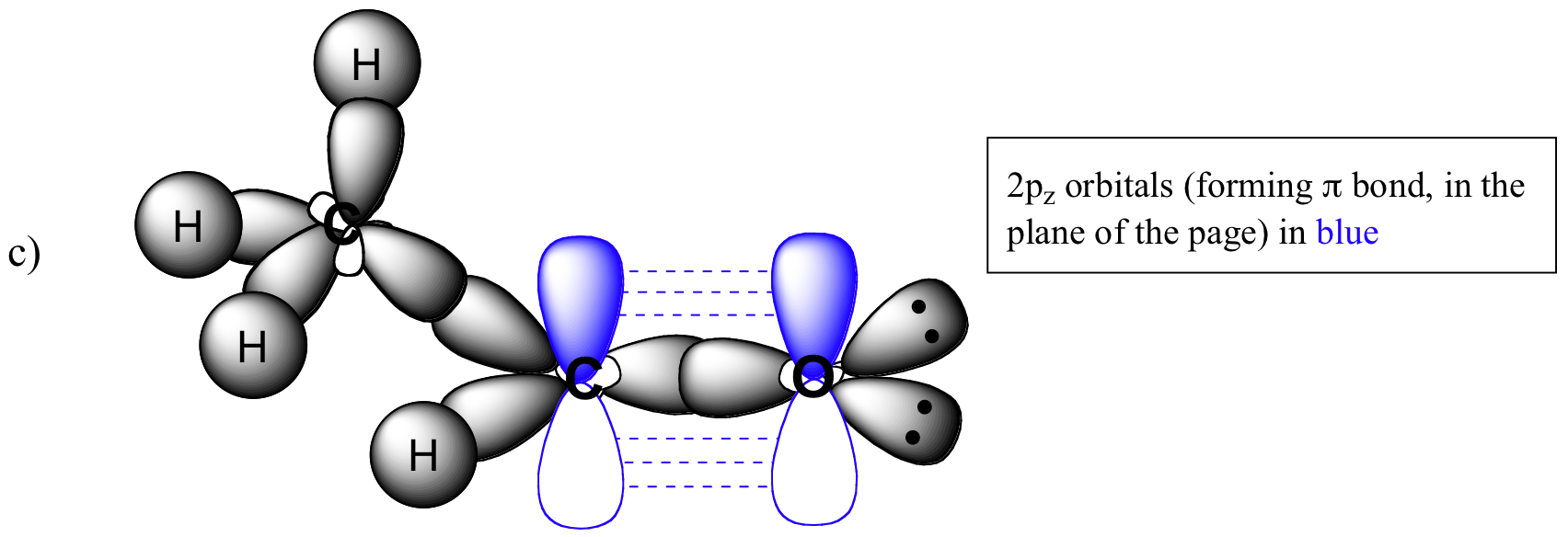
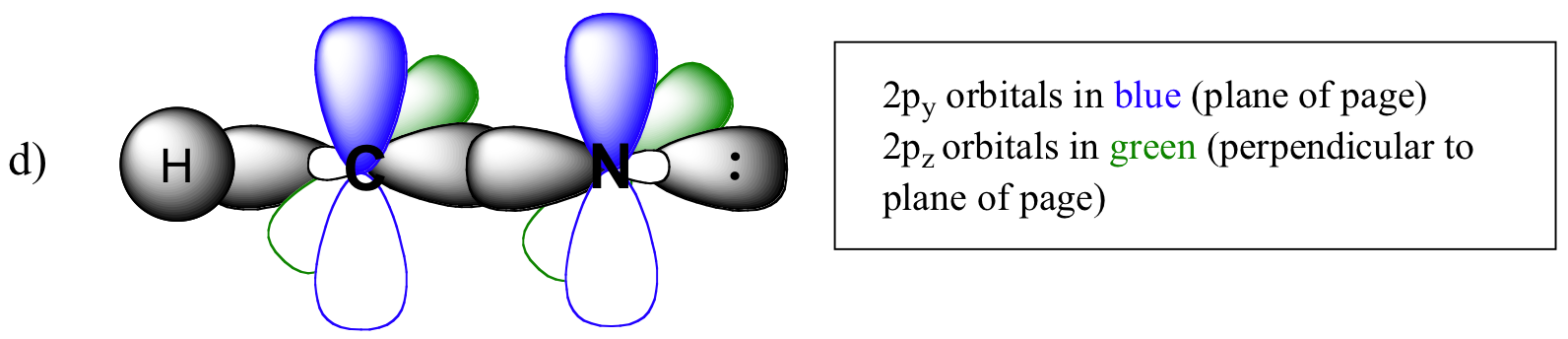
P1.2:
- a lithium cation (Li+): 1s2
- a calcium cation (Ca2+): 1s22s12p63s23p6
- a iron cation in the ferric (Fe+3) state: 1s22s12p63s23p64s23d3
P1.3: There are many possible correct answers to this problem – check your answer with your TA or instructor.
P1.4:
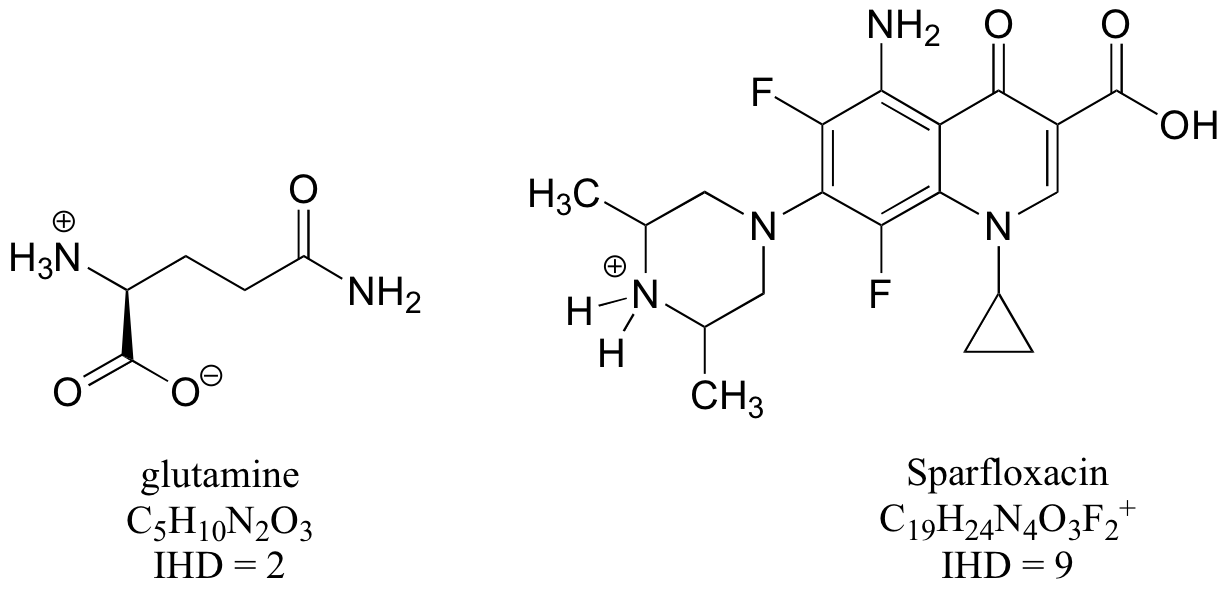
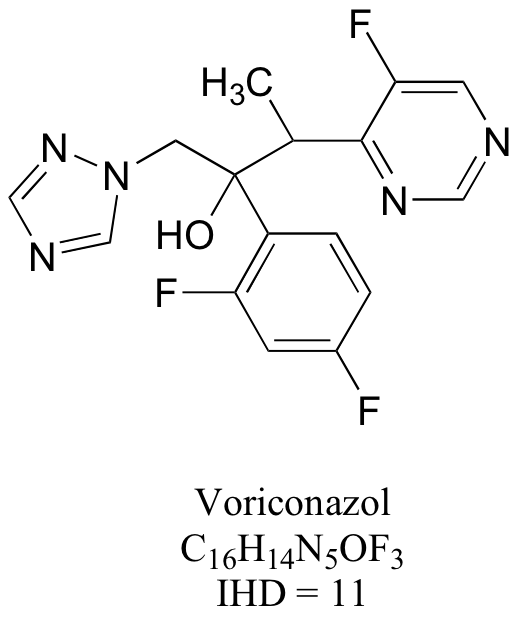
Notice in doing this problem that it is much easier to determine the IHD from the structure rather than the molecular formula – just count rings and multiple bonds! But it would still be good practice to use the molecular formulas to calculate IHDs, then check that they match what you determined from the structures.
P1.5:
a-i; j) see structures below
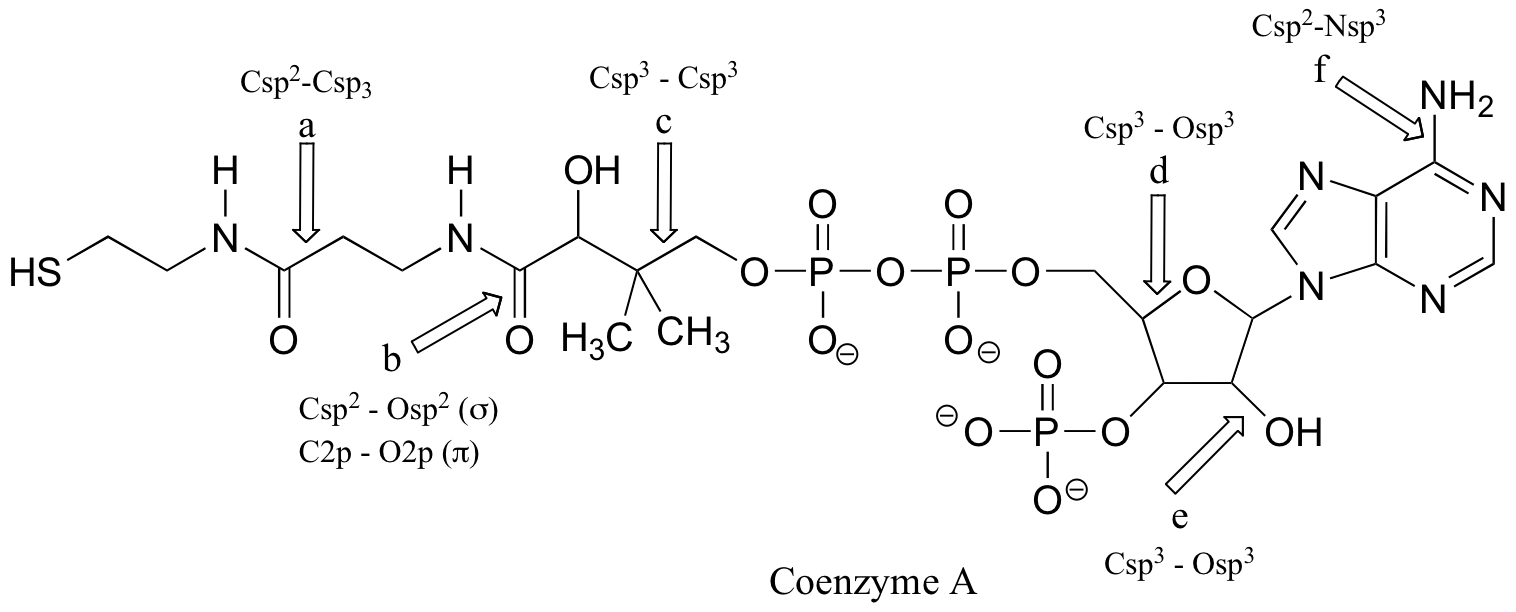
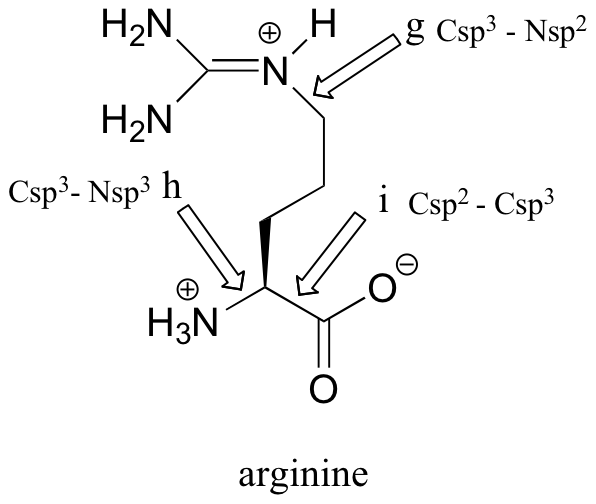
k) the molecular formula of arginine is C6H15N4O2.
P1.6:
a) Csp3 – Osp3
b) Csp2 – Csp3
c) Csp2 – Nsp2
d) Csp2 – Csp2
e) Csp3 – Csp3
f) Csp2 – Csp2
g) Csp3- Csp3
h)Csp2 – H1s
i) Csp2 – Osp2
j) Csp2 – Cl3p
k) Nsp3 – H1s
l) Compound 3 contains two aldehydes, compound one contains an ether, compound 2 contains an amide, compound 3 contains a terminal alkene, and compound 4 contains a secondary amine.
m) The molecular formula of compound 3 is C10H14O2.
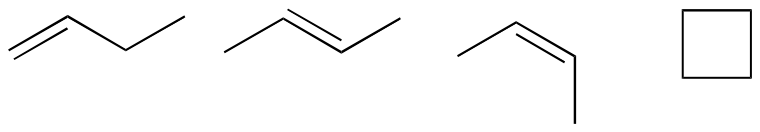
P1.7:
P1.8:
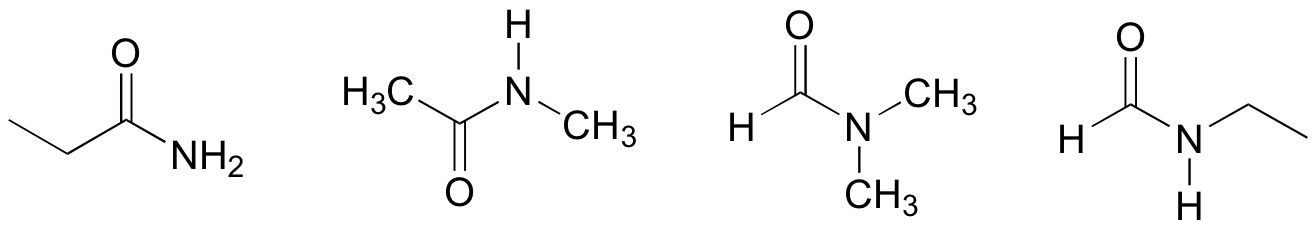
P1.9: shortest e<c<d<f<b<a longest
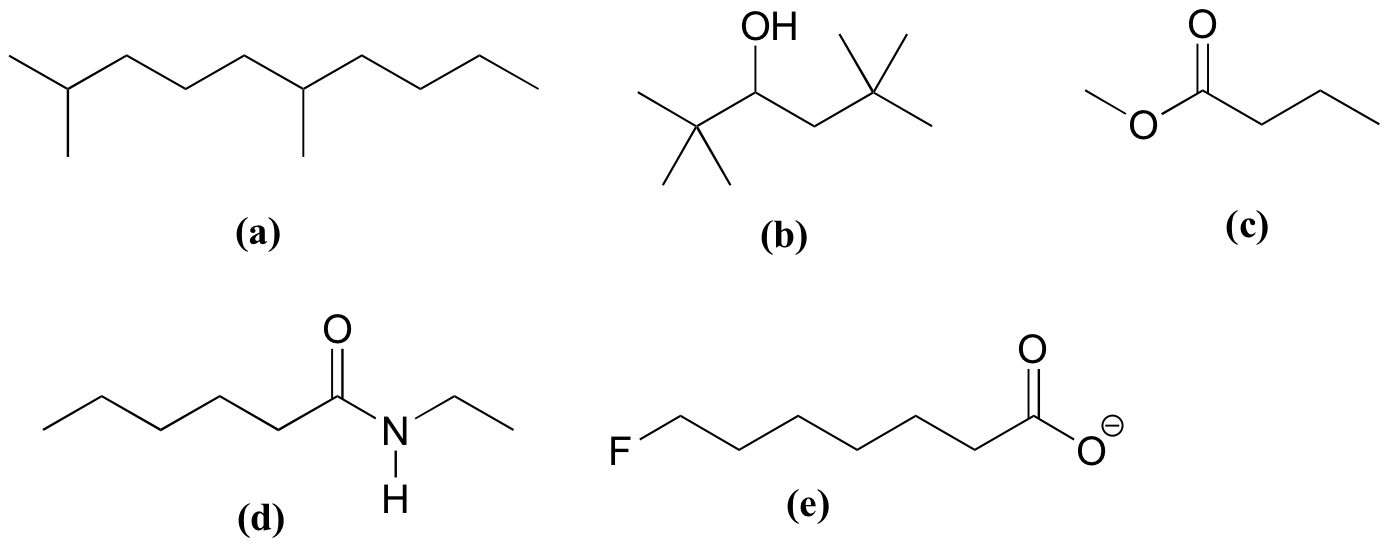
P1.10
Challenge problems
C1.1: If the bonds in water were formed from the two half-filled, unhybridized 2p orbitals on oxygen, the bonding geometry would be approximately to 90o, rather than 103.5o as is actually observed. Unhybridized p orbitals lie at right angles to one another!
C1.2: The bonding an allene can be depicted thus:
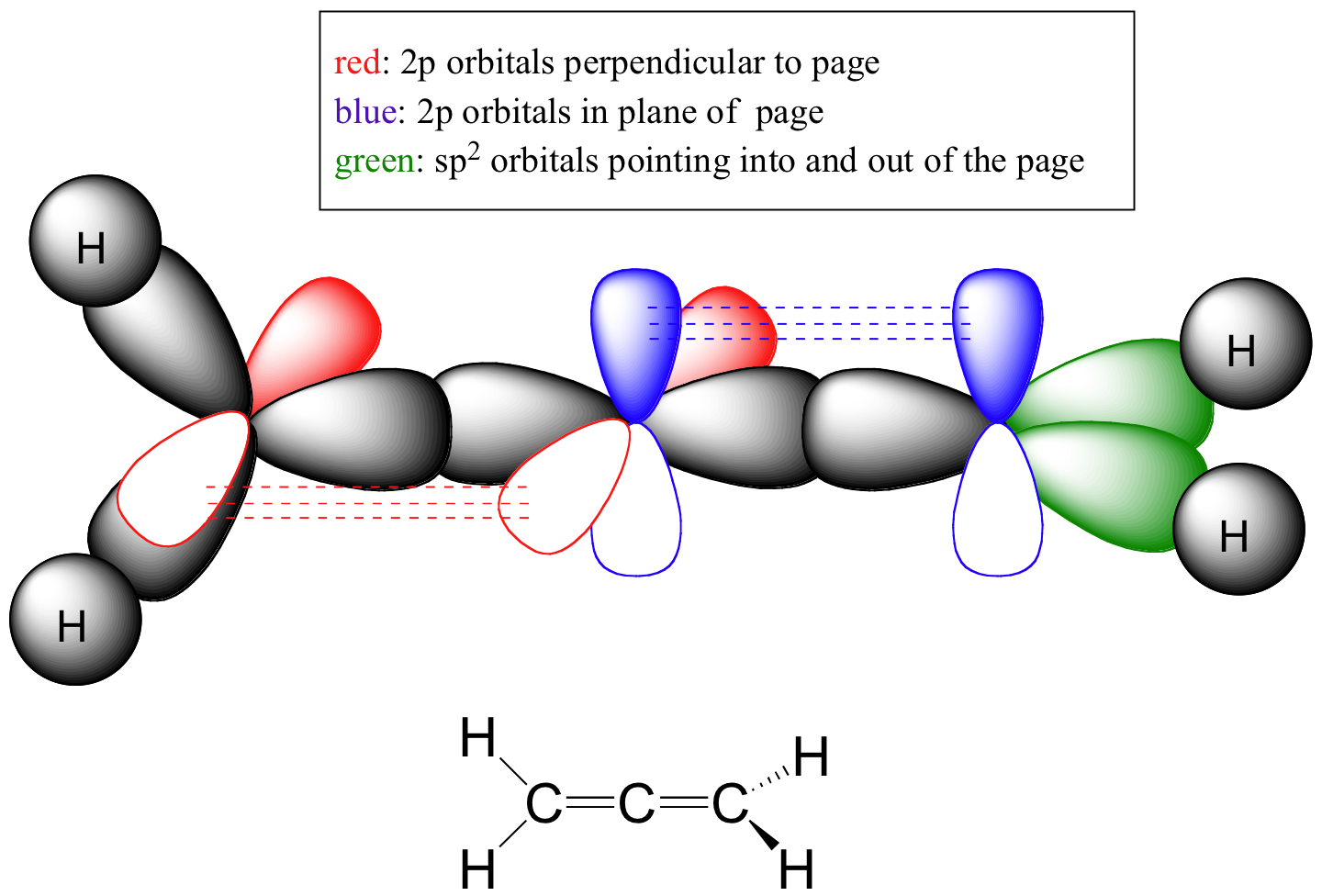
Notice that the central carbon is sp-hybridized, while the two end carbons are sp2-hybridized: the left one with the three sp2 hybrid orbitals in the plane of the page, the right one with two if the three orbitals pointing into or out of the plane of the page.
Contributors
- Organic Chemistry With a Biological Emphasis, Tim Soderberg (University of Minnesota, Morris)


