Extra Credit 65
- Page ID
- 49909
Q2.50
What concept (microscopic or macroscopic) best describes temperature?
Q2.73
Determine the mean free path of F2 molecules with a collision diameter of 4.25 angstrom. The following conditions apply to the experiment; temperature of 400 K and a total pressure of \(2 \times 10^{-10}\; torr\).
Q2.95
Scientists have recently discovered a new planet. Planet Sal has a universal gravitational constant (G) of \(9.23 \times 10^{-18}\; m^{3}\; kg^{-1}\; s^{-2}\; \), mass (M) of \(8.5 \times 10^{21}\; kg^{-1}\; \), and a radius of \(8.6 \times 10^{8}\; m\; \). Escape velocity(v) can be determined with the following equation: v=(2GM/ r)1/2. The thermosphere of planet Sal contains two molecules (H2 and Fluorine) with an altitude of 200Km and 324K. Compare the average speed of both molecules and determine which molecule has a lower tendency to escape.
Q9.12
\[2CO_{2 (g)} \rightarrow 2CO_{(g) }+ O_{2 (g)}\]
The above reaction is first order with a rate constant of 0.78 s-1. The reaction takes place at a temperature of 200K. Calculate in units of seconds the amount of time it takes for the concentration CO2 to increase from 0.15M to 0.74M.
Q10.2
Based on the following values determine whether the ES (Enzyme-substrate) displays steady state or equilibrium model.
Relevant information:
k1= \(6 \times 10^{9}\; M^{-1}\; s^{-1}\; \)
k-1= \(5 \times 10^{7}\; s^{-1}\; \)
k2= \(4 \times 10^{2}\; s^{-1}\; \)
Q11.2
In order to remove an electron from a metal surface requires a starting point frequency of \(9.23 \times 10^{22}\; Hz\; \). Using the information provided, determine the energy that would remove an electron from a metal.
Q11.26
Derive the equation E= hv by demonstrating its connection to the Broglie's equation.
Hint: Think about the wavelength in terms of a particle in a box with a nth level
Q12.9
Phosphine (H3P) is an acidic compound that loses three protons. The ion (P-3) is formed by completely protonating the compound. Using the molecular orbital theory describe the bonding scheme of P-3. Calculate the bond order P-3 and P. State whether it is paramagnetic or diamagnetic.
Q14.1
Use the value 30, 500 m-1 and convert it to frequency and wavelength values.
Q14.24
Show all the vibrational modes of the following molecules.
a) CO2 b) OF2 and identify whether the two molecules are IR active.
Answer Key
Q2.50: Macroscopic concept
Q2.73: 339.65 m
Q2.95:
Average speed for (H2) = \(2.39 \times 10^{-9}\; m/s\)
Average speed of (F) = \(7.74 \times 10^{-10}\; m/s\)
H2 would have a lower tendency to escape.
Q9.12: 2.05 s
Q10.2: Equilibrium model
Q11.2: \(6.1158 \times 10^{-11}\; J\)
Q11.26: E= 1/2 mv2= 1/2m (n2h2/4m2L2)= n2h2/8mL2
Q12.9: The P-3 has 8 electrons, which can be arranged in energy levels described by molecular orbital theory. Two electrons occupy the sigma 2s orbital and another two electrons occupy the antibonding sigma 2s orbital. Then the next two electrons occupy the sigma 2pz orbital. From there the pi 2px and 2py levels are next. Of the remaining two electrons, one electron fills the pi 2px and pi 2py. This scheme makes P-3 paramagnetic.
Bond Order (P-3)= 2 ; Bond Order (P)= 1/2 ; both paramagnetic
Q14.1: wavelength: \(3.28 \times 10^{-5}\; m\)
frequency: \(9.14 \times 10^{12}\; s^{-1}\; \)
Q14.24: The image below describes the vibrational nodes for both CO2 and OF2
image was used from Google images:
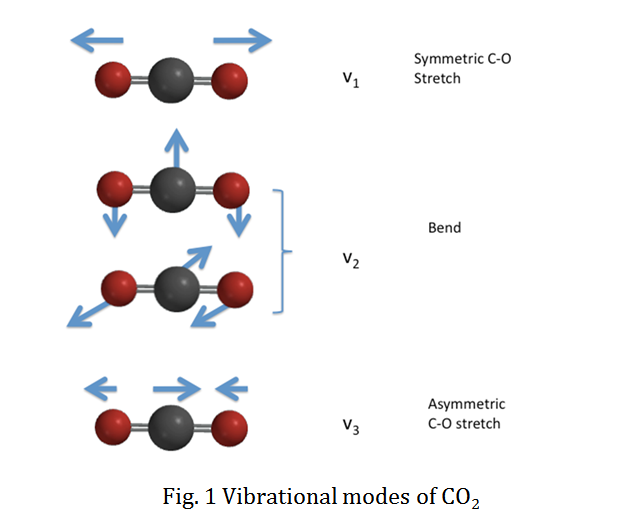
CO2 and OF2 are not IR active
Solutions
Q2.50: Temperature is a macroscopic concept, since the average kinetic energy of a system depends on temperature based on the kinetic theory of gases. The equation below displays how temperature influences the kinetic energy.
\[KE = \dfrac{1}{2}mv^2 = \dfrac{3}{2}k_BT\]
Kinetic theory of gases consists of the assumption that is deals with a large number of molecules, making the molecules a microscopic concept and temperature the restriction in the system.
Q2.73: Use the free mean path equation to solve for the the free mean path value.
\
Step 1. Gather the information given in the problem
d represents the diameter (m)
N represents number of molecules
V represents the volume
The problem provided diameter (angstrom), temperature (K), and total pressure.
d=4.25 angstrom which need to be converted to meters (1 angstrom = \(1 \times 10^{-10}\; m\; \)
T=400K
P= \(2 \times 10^{-10}\; torr\) which needs to be converted to atm units (1torr=1atm)
Step 2. Use the ideal gas law equation \[ PV=nRT \] to solve for \[\dfrac{N}{V}\]. Since we are solving for N not n we must multiply the equation by avogadro's number (NA). Manipulate the equation to solve for \[\dfrac{N}{V}\]
\[\dfrac{N}{V} = \dfrac{PN_{A}}{RT}\]
(N/V)=\(3.669\times 10^{12}\; L^{-1}\; \)
multipy this value by (1000L)/(1m3) to get \(3.669 \times 10^{15}\; m^{-3}\; \)
Step 3. Plug in the values calculated to find the mean free path
Free mean Path=339.65m
Q2.95: To solve for the average speed of both particles use the average speed equation.
Kb=\(1.3806 \times 10^{-23}\;J\; K^{-1}\; \)
T=324K
m (H2) =2 g/mol
m (F)= 19 g/mol
The mass of the particles need to be converted to Kg to allow units to be canceled out.
Plug in the values to the equation and you should get;
Average speed for (H2) = \(2.39 \times 10^{-9}\; m/s\)
Average speed of (F) = \(7.74 \times 10^{-10}\; m/s\)
Q9.12: Since the problem states the reaction is first order, we use the equation below.
To determine the amount of time the reaction takes the rate law must be integrated.
\[ \ln{\dfrac{[A]_t}{[A]_0}} = -kt \]
rearrange the equation to solve for t and plug in the values below;
rate constant is (K)=0.78 s-1
the initial concentration is 0.15M and the concentration [A] is 0.74M
t=2.05 s
Q10.2:
In order to determine whether the enzyme substrate displays equilibrium or steady state you must compare the Km and Ks.
Km= (k-1+k2)/(k1)
Ks=(k-1)/(k1)
Based on the values of Ks and Km, it can be determined that the reaction displays a equilibrium model since Km=Ks.
Q11.2:
To solve for the energy required to remove an electron from a metal we use the planck's equation: E=hv
Frequency was given v=\(9.23\times 10^{22}\; s^{-1}\; \) and h is planck's constant \(6.626\times 10^{-34}\; s^{-1}\; m^{2}\; kg\; \)
Plug the values into the equation; E= \(6.1158 \times 10^{-11}\; J\; \)
Q11.26
Planck's equation
\[E = \dfrac{h}{v} = \dfrac{hc}{ \lambda}\]
Broglie's equation:
\[ \lambda = \dfrac{h}{p}\]
\[ \lambda = \dfrac{h}{mv}\]
We know that \[ \lambda = \dfrac{2L}{n}\] for a particle in a box with nth level.
Knowing this \[ \dfrac{2L}{n} = \dfrac{h}{mv}\]
\[ v = \dfrac{nh}{2mL}\]
Now input the above equation to replace v in the Energy equation below. This will show the connection between equations.
\[ E = \dfrac{1}{2} \times mv^{2}\; = \dfrac{1}{2} \times m \dfrac{n^2 h^2}{4m^2 L^2}\; = \dfrac{n^2 h^2}{8mL^2}\]
Q12.19: To calculate the bond order for P-3 and P you need to calculate the electrons for each one. Once the electrons have be determined you proceed to filling out the Molecular Orbital diagram. This diagram will help calculate the number of electrons on antibonding orbitals versus bonding orbitals, which is information needed to calculate the bond order. The completed diagram also helps decide whether it is paramagnetic or diamagnetic.
\[ bond\; order=\dfrac{number\; of \; bonding\; electrons-number\; of \; antibonding\; electrons}{2} \]
Since P-3 has a total of 8 electrons it would fill the sigma 2s bonding orbital with two electron, the sigma 2s antibonding orbital (2e-), the sigma 2p bonding orbital (2e-), the for the pi 2px and 2py bonding orbitals 1 electron goes on each, making it paramagnetic. There is a total of 6 electrons on bonding orbital and 2 on antibonding orbitals. 6-2=4/2=2
For P it has five electrons that fill the sigma 2s bong and antibonding orbitals (4e-), and 1e- on the sigma 2pz bonding orbital. It is paramagnetic and has a bond order of 1/2
Q14.1: The value given is the wavenumber. Frequency and wavelength can be determined from the wavenumber by using the following equation:
\[wavenumber = \dfrac{1}{\lambda} = \dfrac{v}{c}\]
\[ \lambda = \dfrac{1}{wavenumber}\]
\[ \lambda = \dfrac{1}{30500}\]
wavelength: \(3.28 \times 10^{-5}\; m\)
Now for frequency
\[ v = \dfrac{c}{\lambda}\]
frequency: \(9.14 \times 10^{12}\; s^{-1}\; \)
Q14.24
The vibrational modes for CO2 and OF2 are the same.
In order to determine whether molecules are IR active the molecule must have a dipole. Symmetric molecules are not IR active since they do not have polar bonds.
CO2 and OF2 are symmetric molecules which therefore means that they are not IR active.

