Extra Credit 63
- Page ID
- 49903
Q2.71
As the workers harvest the oranges, they toss them into a sack with a volume of 6.24m3. Each sack can hold up to 150 oranges if each orange has a diameter of 7 cm. Calculate the mean free path of the oranges.
Q2.81
Some scientists want to purify iridium - 192 from iridium - 188. They miraculously found that they were able to vaporize iridium with iodine to form IrI4. Assuming the starting mixture was at a 50:50 ratio of iridium - 192 to iridium - 188, after 3 stages of separation, what would be the percentage of enrichment?
Q9.10
Potassium chlorate can decompose into chlorate and O2 in the equation:
\[2KClO_{3 (g)} \rightarrow 2KCl_{(g) }+ 3O_{2 (g)}\]
The reaction was allowed to proceed and the concentration of potassium chlorate was measured over time. From the data, determine the order of the reaction and the rate constant.
Hint: Graph the following data to help you determine the order of the reaction.
| KClO3 | 372 | 259.5 | 181.0718 | 126.83 | 88.14 |
| Time(s) | 0 | 1500 | 3000 | 4500 | 6000 |
Q9.18
Nuclear fuel that has been used up contains plutonium - 238, which can decay to uranium - 234 in the reaction below:
Pu94238 → U92234 + He24 t1/2 = 87.7 years
If the amount of plutonium was initially 42 moles, calculate the grams of uranium after 263.1 years.
Q9.35
At the temperature 1102oC, the rate constant for converting cyclobutane to butene is 7.42 x 10-6 s-1. Calculate the Delta G of the transition state of this process.
Hint: rate = k = vk' where v =kbT/ h and k' = e(-G' / RT)
Q10.09
A substance that is known to be an inhibitor is added to a solution containing an enzyme, but the nature of the inhibition is unknown. What are the three possible types of inhibition that it could be, and how can each type be distinguished from each other. How does Vmax and Ks change with increasing inhibitor concentration?
Q11.24
In the equation, En= n2h2/ 8mL2 the energy is inversely proportional to the mass. What is the dependence of mass in terms of the Heisenberg uncertainty principle?
Q12.17
Use MO Theory to describe the bonding in O2+, O2, O2-. What are the bond order for each molecule? Describe the trend of bond energy and bond lengths.
Q13.19
What techniques can be used to detect hydrogen bonds?
Q14.22
Define the zero-point energy and explain the reasoning behind the zero-point energy.
ANSWERS
Q2.71) 1.966 m
Q2.81) 0.86%
Q9.10) First order. k = 0.00024 s-1
Q9.18) 1249.5 g
Q9.35) 489,304.8 J mol-1
Q10.09) competitive, uncompetitive, non-competitive; look at the graph of 1/Vo Vs. 1/[S]. ; For competitive, Vmax does not change, while Ks increases; for uncompetitive, Vmax decreases and Ks decreases; for non-competitive, Vmax decreases, while Ks is unchanged.
Q11.24) As mass of the particle decreases, the uncertainty of the particle decreases. A decrease in mass leads to a decrease in momentum, ∆p, in the Heisenberg uncertainty equation, which causes the length of the box, ∆x, to increase because the uncertainty has to be greater than or equal to h/π*4. Increase in length means decrease in energy according to the equation for En.
Q12.17)
For O2+, (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)1; For O2 (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)1(πy*)1
For O2-, (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)2(πy*)1.
O2+ = 2.5; O2 = 2; O2- =1.5
Increasing bond order means stronger bonds and shorter bond length.
Q13.19) X-Ray crystallography, Infrared spectroscopy, and Nuclear Magnetic Resonance Spectroscopy.
Q14.22) The lowest possible energy state. It can not be 0, because molecules are always in motion.
SOLUTIONS
Q2.71
To solve this problem, you have to use the equation: λ = 1/( sq(2) * π * d2 * nv),
where
- "d" is the diameter of the molecule (in this case oranges)
- nv is the density of the molecules
To find nv, divide the number of molecules by the volume
150/6.42
= 23.36
From this point, plug in the numbers into the equation. Make sure to convert the 7cm to 0.07m
λ = 1/( sq(2) * π * d2 * nv)
λ = 1/ ( sq(2) * π * 0.072 * 23.36)
λ = 1.966
Q2.81
First, you have to solve for the separation factor, s, which is found through
s = Rate of effusion of 188IrI4/ Rate of effusion of 192IrI4
The ratio of the rates of effusion is equal to the square root of the inverse ratio of the molecular weights of the molecules
Rate of effusion of 188IrI4/ Rate of effusion of 192IrI4 = Molecular weight of 192IrI4/ Molecular weight of 188IrI4
The molecular weight 192IrI4 is 192 + (4 * 127) = 700. 127 is the molecular weight of iodine.
The molecular weight 188IrI4 is 188 + (4 * 127) = 696.
So,
s = Sqrt(700/696)
s = Sqrt(1.005747)
s = 1.002869
Since this process occurs three times, you must take the separation factor and raise it to the exponent of 3.
1.0028693
= 1.008633
This number tells us the total percent of separation of 192IrI4 from 188IrI4 after three stages. So, since we're looking at just the percentage of enrichment, subtract the separation factor by 1 to account for the percentage of 192IrI4 that we started with and multiply by 100 to find the percentage of enrichment.
Q9.10
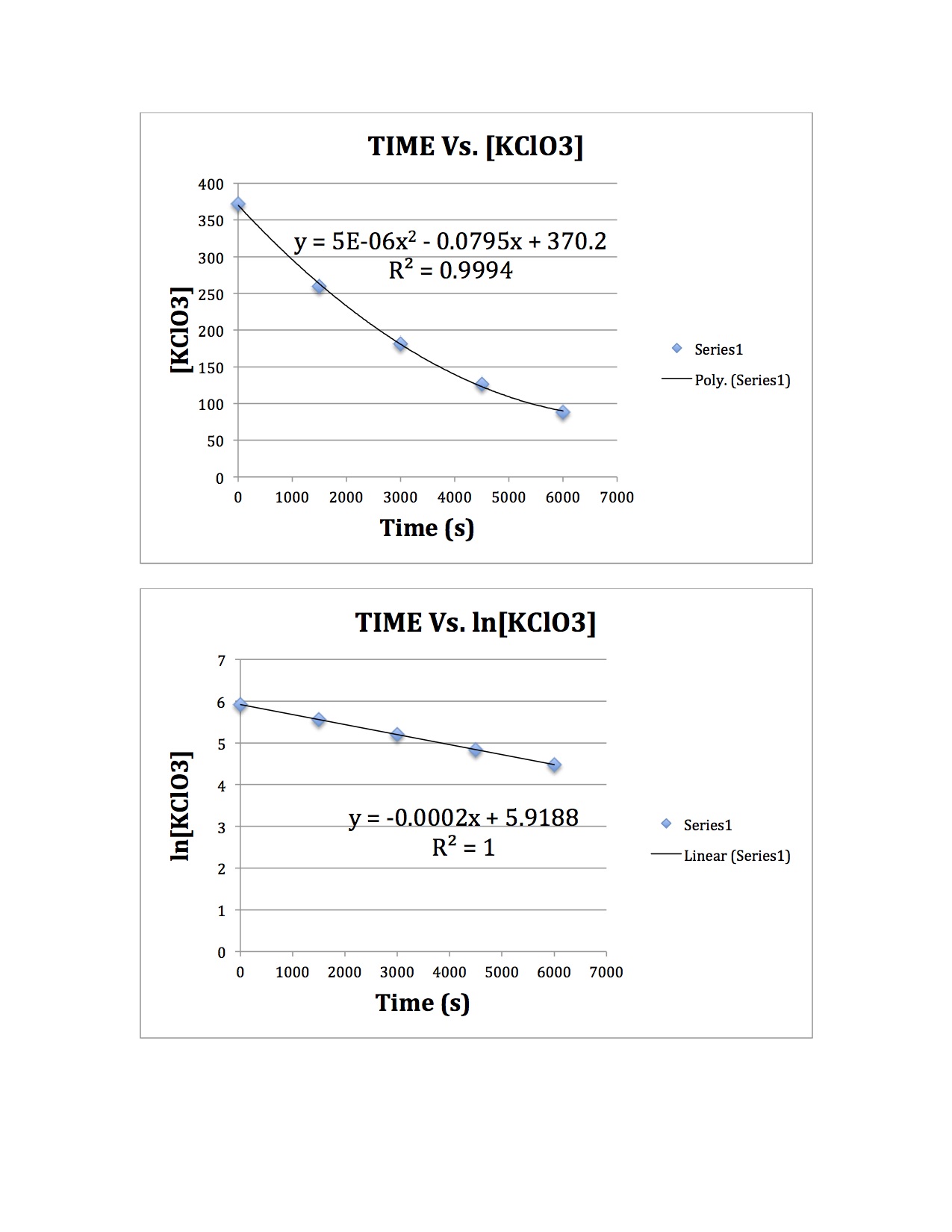
First, graph the data in a program to see and look at the shape of the graph. If the plot of Time Vs. [KClO3] turns out to be linear, then the reaction is of the 0th order. Since in this case the graph is not, the data must be graphed to see if it is 1st or 2nd order.
To see if the plot is of 1st order, a Time Vs. ln[KClO3] plot must be made. Since this plot is linear, the reaction is of the first order and we do not need to test if to check if it is of 2nd order.
To find the rate constant, just look at the slope of the equation of the line. The rate constant is the absolute value of the slope, so it is approximately, 0.0002. Graphs are displayed below.
An alternative method to determine the rate constant involves deriving the first order rate equation: d[A]/dt=k[A]. If we were to derive the equation then it would be In[A]-In[Ao]=kt. Based on the table of information given with concentration and time the equation can be manipulated to solving for k (rate constant): k=(In[A]-In[Ao])/(t). [Ao] is the initial concentration (t=0) based on the table above, while [A] can be any of the concentration values after the initial. I decided to use (t=6000) with a [A]=88.14.
Answer: In(88.14) - In(372) / (6000-0) = k =0.0002
Q9.18
To start this problem, notice that the half-life of the decay is 87.7 years and the time waited is 263.1 years. 263.1 divided by 87.7 is 3, so 3 half-lives would have passed.
So, then we divide the amount of moles we started with by 2 three times, or just divide by 8 (23), to get 5.25 moles. this is how much plutonium is leftover.
The amount of uranium acquired after the time would be 42 - 5.25 = 36.75 moles.
To find the grams of uranium, multiply the moles of uranium acquired by the molecular weight of uranium to get the grams of uranium.
36.75 mole * 234 g/mole
= 8599.5 grams
Q9.35
Start of with the equation:
k = ((Kb * T) * e-G'/RT)/h
where
- "k" is the rate constant,
- Kb is boltzmann's constant
If we rearrange the equation to solve for ∆Go, then we get the equation:
∆Go = -R * T * ln((k * h)/ Kb * T)
Convert the temperature to kelvin and plug the numbers in
∆Go = -8.147 J mol-1 K-1 * 1375.15 K * ln((7.42 x 10-6 s-1 * (6.62607004 × 10-34 m2 kg / s )/ 1.38064852 × 10-23 m2 kg s-2 K-1 * 1375.15 K)
∆Go = 489,304.8 J mol-1
Q10.09
The three types of inhibition are competitive, uncompetitive, and non-competitive. To tell them apart, it is best to look at the lineweaver-burk plots of each time of inhibition. For competitive inhibition, the addition of inhibitor increases the slope of the graph; this increases the Ks app but the Vmax is constant. For uncompetitive inhibition, both the Vmax and the Ks decrease. On the lineweaver-burk plot, the line of the graph should rise up vertically. For the non-competitive inhibitor, the Vmax decreases, but the Ks stays constant. On the lineweaver-burk plot, the addition of inhibitor makes it appear that all the lines graphed emanate from one point on the negative X-axis.
Q11.24
As mass of the particle decreases, the uncertainty of the particle decreases. A decrease in mass leads to a decrease in momentum, ∆p, in the Heisenberg uncertainty equation, which causes the length of the box, ∆x, to increase because the uncertainty has to be greater than or equal to h/π4. An increase in length means a decrease in energy according to the equation for En.
Q12.17
Add the total amount of electrons of O2 and add it to the molecular orbital diagram using Hund's rule and the Pauli exclusion principle. For oxygen, the sigma bonds are under the π bonding orbitals. Subtract or add the electron according to the molecule, and add it to the diagram.
For O2+, (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)1
For O2 (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)1(πy*)1
For O2-, (σ1s)2(σ1s)2(σ1s)2(σ1s)2(σ1s)2(πx)2(πy)2(πx*)2(πy*)1.
The bond orders for the molecules are: O2+ = 2.5; O2 = 2; O2- = 1.5
As for the trend, with increasing bond order means the bond strength increases. Since the bonds are stronger, the distant between the atoms are shorter since they pull each other closer, and the energy to break the bonds becomes much higher.
Q13.19
The techniques that can be used to detect hydrogen bonds include X-Ray crystallography, Infrared spectroscopy, and Nuclear Magnetic Resonance Spectroscopy.
Q14.22
The zero-point energy is the lowest vibrational energy state that a molecule can achieve. The lowest energy state is not 0, but rather (1/2)hv, where v represents frequency. The reason behind the zero-point energy is that molecules are always in motion even at absolute zero, so they technically have some energy still.