Extra Credit 55
- Page ID
- 49895
2.58)
Which of the atoms will give the greatest 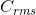 at 30°C? a) He b) C c) O? Solve this problem by using the equation for
at 30°C? a) He b) C c) O? Solve this problem by using the equation for 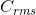 but without calculation.
but without calculation.
Solution:
crms = vrms = \(\sqrt{\frac{3RT}{M}}\)
The smaller the molar mass, the greater the .gif?revision=1&size=bestfit&width=36&height=15) , so He has the greatest root-mean-square speed (Crms).
, so He has the greatest root-mean-square speed (Crms).
9.2)
The rate law for the reaction:
.gif?revision=1&size=bestfit&width=333&height=20)
is given by rate = k [NH4+][NO2-]. At 25ºC, the rate constant is 3.0 x 10-4 M-1s-1. Calculate the concentration of [NH4+] if the rate is 7.23 x 10-6 M s-1 and [NO2-] = 0.03M.
Solution:
rate = k [NH4+][NO2-]
7.23 x 10-6 M s-1 = (3.0 x 10-4 M-1s-1)(0.03M)[NH4+]
[NH4+] = 0.8033M
9.26)
For reaction:
\[O_3 + O \rightarrow 2O_2\]
\[Rate= k{\frac{[O_3]^2}{[O_2]}}\]
Calculate the activation energy at 350K, 500K, and 1000K with the rate constant 3 x 109S-1. Assume A = 1010S-1 in each case.
Solution:
\[k=Ae^{\frac{-E_a}{(R)(T)}}\]
At 350K:
\[3·10^9=10^{10}e^{\frac{-E_a}{(8.314)(350K)}}\]
Ea = 3503 Jmol-1
At 500K:
\[3·10^9=10^{10}e^{\frac{-E_a}{(8.314)(500K)}}\]
Ea = 5004 Jmol-1
At 1000K:
\[3·10^9=10^{10}e^{\frac{-E_a}{(8.314)(1000K)}}\]
Ea = 10009 Jmol-1
10.1)
What assumption is made differently in between the rapid equilibrium and the steady state kinetics?
Solution:
Rapid equilibrium assumes k-1>>k2, so the enzyme complex is dissociating faster than product. Steady-state kinetics assumes
k2 >>k1, so the enzyme complex is forming at the same rate as it is turning into product.
11.16)
The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17J. For light of 320 nm, how many photons does this correspond to?
Solution:
\[E={\frac{hc}{\lambda}}\]
\[E={\frac{(6.626·10^{-34})(3·10^8)}{320·10^9}}\]
\[E=6.212·10^{-19}J\]
\[64 photons={\frac{4.0·10^{-17}}{6.21·10^{-19}}}\]
12.7)
Consider the following Lewis structure for 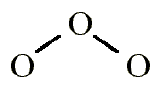 . Does it satisfy the octet rule? If not, draw additional resonance structures that do satisfy the octet rule.
. Does it satisfy the octet rule? If not, draw additional resonance structures that do satisfy the octet rule.
Solution:
It doesn't satisfy the octet rule because it is missing lone pairs of electrons on each oxyge. The following resonance structrues satisgy the octet rule:
13.11)
Calculate the bond enthalpy of KCl using the Born-Haber cycle. The bond length of KCl is 212pm. The first ionization energy is 418.KJ mol-1 and the electron affinity for Cl is 349 KJ/mol. Use n = 10.
Solution:
\[V_o={\frac{q_+q_-}{4\pi\varepsilon_or_e}}(1-{\frac{1}{n}})\]
\[V_o={\frac{(1.602·10^{-19})^2(6.022·10^{23})}{4\pi(8.854·10^{-12})(212·10^{-12})}}(1-{\frac{1}{10}})\]
\[V_o=5.897·10^5N·m/mol\]
\[V_o=589.7KJ/mol\]
\[-D=I_1-E_A+V_o\]
\[-D=418.71-349+(-589.7)=-520KL/mol\]
14.4)
Convert the following percent transmittance to absorbency: a) 80% b) 35% c) 100%
Solution:
\[-log(T)=A\]
\[A)-log(0.80)=0.097\]
\[B)-log(0.35)=0.46\]
\[C)-log(1)=0\]
9.9)
The progress of a reaction in the aqueous phase was monitored by absorbance of a reactant at the following times:
| time(s) | 0 | 54 | 171 | 390 | 720 | 1010 | 1190 |
| Absorbance | 1.34 | 1.25 | 1.07 | 0.807 | 0.526 | 0.360 | 0.285 |
Assuming 1st order kinetics, find the rate constant.
Solution:
\[ln{\frac{[A]}{[A_o]}}=-kt\]
\[ln{\frac{[1.25]}{[1.34]}}=-k(54s)\]
\[k=0.0013s^{-1}\]
2.70)
How does the molecular collisions depend on a) the average speed b) the number of molecules c) the volume d) the separation between the 2 spheres?
Solution:
.gif?revision=1&size=bestfit&width=152&height=41)
a) directly proportional
b) directly proportional
c)inversely proportional
d)directly proportional