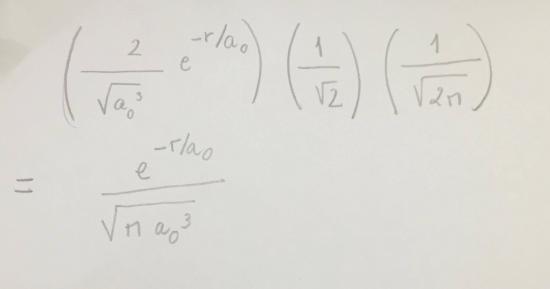Extra Credit 46
- Page ID
- 49207
Q2.10
Nitrogen is one of gases that filled up the air in a tire. Assume the vapor pressure of nitrogen gas is 0.00018 mmHg at 298 K and density of air inside a tire is 1.22 g/L.
-
Using Ideal Gas Law to calculate the concentration of nitrogen gas contained in that tire.
- What is the number of parts per million (ppm) by mass of nitrogen gas in a tire's air?
S2.10
Ideal Gas Law: PV= nRT
1. Concentration = n/V = P/ RT = ((0.00018 mmHg) (1atm/ 760 mmHg)) / (0.08206 L atm/ K mol) (298K) = 9.69 x 10^-9 mol/L
2. In 1L, n (N2) = 9.69 x 10^-9 mol
m (N2) = 9.69 x 10^-9 mol x 28.0 g/mol = 2.71 x 10^-7 g
m (air) = 1.22 g
m (total) = m (air) + m (N2) = 1.22 g + 2.71 x 10^-7 g = 1.22 g
ppm of N2 in tire's air = [m (N2) / m (total) ] x 10^6 = (2.71 x 10^-7 g / 1.22 g) x 10^6 = 2.22 x 10^-1
Q2.56
What is the root-mean-square speed of Argon (Ar) at 475 K?
S2.56
$$c_{rms} =\sqrt{\frac{3RT}{M}}$$
$$=\sqrt{\frac{(3(8.314 JK^{-1}mol^{-1)}(475 K)}{39.95KgX10^-3 kgmol^1}}$$
$$=545 ms^{-1}$$
Q2.80
In 3 minutes, 30.0 ml He effuse through a small hole. With the constant temperature, pressure, and the same time 10.0 ml of a mixture N2 and N2O effuse. What is the percent composition by volume of the mixture?
S2.80
Use Graham's Laws : r1/ r2 = (M2/M1)^1/2
M(mix) = (r CH4 / r mix )^2 x M H2 = ( (30.0/ 3) / (10.0/ 3) ) ^2 x 4.003 = 36.0 g/mol
Let x(N2) be the mole fraction of N2 and X(NO2) be the mole fraction of NO2.
We have x(N2) + x(NO2) = 1
x(N2) M(N2) + x(NO2) M(NO2) = M mix
x(N2) M(N2) + (1 - x(N2) ) M(NO2) = M mix
x(N2) ( 28.0) + (1 - x(N2)) (46 g/mol) = M mix
28 x(N2) + 46 - 46 x(N2) = 36.0
x(N2) = 0.55
At constant P and T, n proportional to V. Therefore, volume fraction = mole fraction.
% of N2 by volume = 55%
% of NO2 by volume = 1- %of N2 = 45%
Q9.17
What is the half-life time for the zero-order reaction if it's 52% complete by 40 seconds?
S9.17
$$For zero-order reaction: [A] = [A]o - kt $$
$$0.48 = 1 - k (40s)$$
$$k = 0.013s^-1$$
$$t_\frac{1}{2}=\frac{[A_o]}{2k}$$
$$\frac{1}{(2)(0.013)}= \frac{1}{0.026}$$
$$t_\frac12=38.46secs$$
Q10.7
An enzyme has a Km value of 5.0 x 10^-5 M, given that the substrate concentration is 0.08M and the initial rate is 2.9 uM/min, what is the initial rate at 3.9x10^-4 M?
S10.7
Step 1: convert uMmin^1 to Ms^1$$
Initial rate is 2.9 uMmin-1=4.83 x 10^-8 Ms-1
$$V_o=\frac{V_{max}[S]}{Km + [S]}$$
$$4.83X10^{-8}=\frac{Vmax(0.08)}{5.0X10^{-5}+0.08}$$
$$V_{max}(0.08)=4X10^{-9}$$
$$V_{max}=V_o=4.83X10^-8 Ms^{-1}$$
When [S] = 3.9 x10^-4 M
$$V_o=\frac{4.83X10^-8)(3.9X10^-4)}{5X10^{-5}+3.9X10^{-4}}$$
$$V_o =4.28X10^{-8}Ms^{-1}$$
Q11.7
How was the temperature on the surface of the sun determined? (Tsun= 5778 K)
S11.7
The surface of the sun is the mean distance from the core at which the light bounces for the last time before it is out. The light we see from the surface has nearly the spectrum of the blackbody. The fraction of light of a Blackbody is emitted at each wavelength is the function of surface temperature alone. Thus, scientists can measure the spectrum of the sun and then fit into a blackbody spectrum to derive the temperature. That's why they got 5778 K for the surface of the sun.
Q11.31
Use Table 11.2as a guide to write the wave function of Hydrogen atom in1s orbital.
S11.31
Q13.2
Arrange the following species in order of increasing melting points: NO, AgCl, H20, C4H10, P
S13.2
$$NO < C_4H_{10} < H_20 < P < AgCl$$
Q14.5
Assuming a chemist estimated the absorption of radiation energy by a molecule. He found out that the absorbance of that sample does not change regardless of time at any wavelength. Explain the result.
S14.5
The absorption of radiation energy by a molecule results in the formation of an excited molecule. When a molecule is excited to a higher state, it often ends up in this lowest excited state and then emits radiation. In this case, the excitation spectrum is the same as the absorption spectrum. Thus, the absorbance remains unchanged.
Q14.28
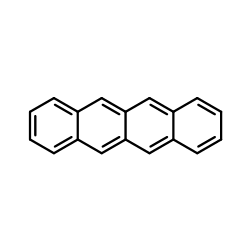
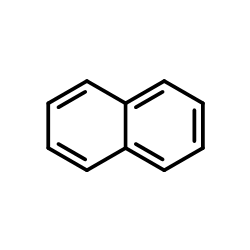
Use what you have learned to explain why Naphthalene is colorless, but tetracene is light orange?
S14.28
Note that in tetracene, the electrons are delocalized over a greater space than those in naphthalene. Based on the particle in a box model, the spacing between energy levels reduce as the length of the box increases; this can be applied here. Tetracene has a bigger "box" causing the absorption wavelength to shift from UV region to the visible region.