Extra Credit 44
- Page ID
- 49205
Q2.54
Determine the average translational kinetic energy for an oxygen molecule at 35°C. Will the value be the same for one mole of the same molecule? If not, solve for it.
S2.54
Average translational energy per molecule will not be the same a average translational energy per mole of the same molecule.
To solve for one molecule
$$E_{trans}=\dfrac32K_BT$$
$$=\dfrac32(1.381 \times 10^{-23}\dfrac{J}{K})(273+35) K=6.38 \times 10^{-21}J$$
To solve for one mole of \(O_2\)
$$E_{trans}=\dfrac32RT$$
$$E_{trans} = \dfrac32 (8.314\dfrac{J}{Kmol})(273+35)K=3.841 \times 10^3Jmol^-1$$
Q2.74
A beaker contains atom X weighing 10gmol-1 moving at 100ms-1. Determine the temperature of the atoms using your understanding of Maxwell distribution.
S2.74
Because all the atoms have the same speed, the total translational energy is
$$E_trans = N(\dfrac12m v^2) = N(E_{trans}) $$
$$N \left( \dfrac12mv^2 \right)= N\left(\dfrac32K_BT\right)$$
$$T={mv^2\over3K_B}$$
$$0.01\dfrac{Kg}{mol}(\dfrac{mol}{6.022 \times 10^{23}})(100ms^{-1})^2\over (3)(1.381 \times 10^{-23}JK^{-1})$$
$$T= 4.01K$$
Q2.100
The molecular weight of a molecule is 30 g/mol. Determine the root mean square velocity at 25°C.
S2.100
$$v_{rms}=\sqrt{3RT\over M}$$
$$\sqrt{3(8.314\dfrac{J}{K\,mol} (273+25\,K)\over 0.03 \dfrac{Kg}{mol}}$$
$$v_{rms}=497.75\dfrac{m}{s}$$
Q9.14.
Given that the integrated rate law for the zero order reaction is $$ [A]=[A]_°-kt $$
- How does the rate depend in the reactant concentration. Explain with words and a graph.
- How does the concentration of the reactants change with time? Explain with words and a graph.
- Derive the half life. Using the derived formula, determine what the half life of the first order reaction depends on.
S9.14
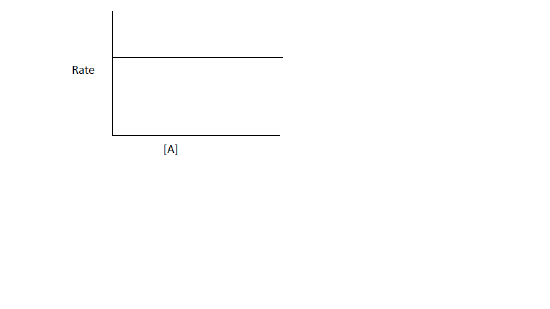
a. The rate law is Rate=k
Thus, the rate of a reaction is independent of the concentration of the reactants.
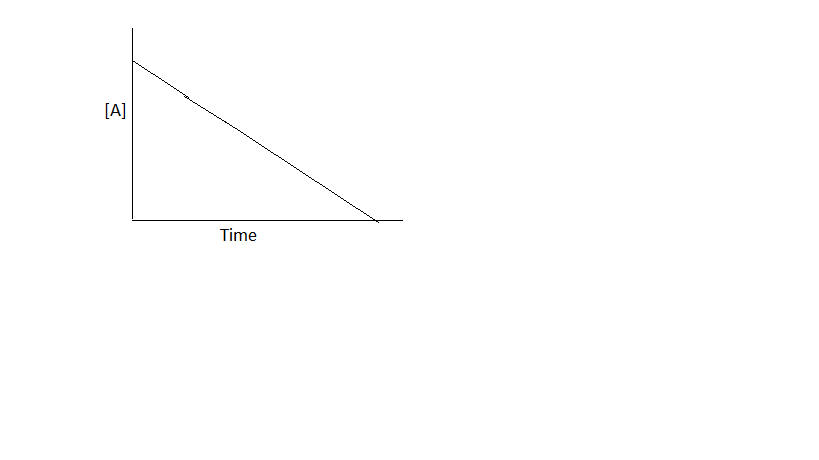
b. As time increases, the concentration of the reactants decrease.
a plot of [A] vs t is a straight line with a slope of -k.
c.At $$t=(t_\dfrac{1}{2})$$
$$Substitute [A]=\dfrac{[A]_o}{2}$$
into the integrated rate equation
$$ [A]=[A]_o-kt$$
$$\dfrac{[A]_o}{2}=[A]_o-K(t_\dfrac{1}{2})$$
$$t_{\dfrac12}=\dfrac{[A]_o}{2k}$$
Q10.4.
Use Michaelis Menten equation:
$$V_o=\dfrac{Vmax[S]}{Km + [S]}$$
to derive
$$\dfrac{V_0}{[S]}=\dfrac{Vmax}{Km}-\dfrac{Vo}{Km}$$
S10.4
step one
multiply both sides of the starting equation by \(K_M+[S]\)
step 2
Divide by \(K_M [S]\), and rearrange
Step 3, solve using algebra
$$Vo=\dfrac{V_{max}[S]}{K_m + [S]}$$
$$V_oK_M + V_o[S]=V_{max}[S]$$
$$V_oK_M=V_{max}[S]-V_o[S]$$
$$\dfrac{V_o}{[S]}=\dfrac{V_{max}}{K_m}-\dfrac{V_o}{Km}$$
Q11.4
Determine the wavenumber, frequency, and wavelength associated with a transition in energy from n= 2 to n= 5 level.
S11.4
Wavenumber and wavelength are inversely related. First, determine the wavelength using the formula below
$$wavenumber= R_Hcm^-1\left(\dfrac{1}{n_i^2}-\dfrac{1}{n_i^2}\right)$$
$$wavenumber=R_H(\dfrac{1}{5^2}-\dfrac{1}{2^2})=23044.77cm^{-1}$$
$$\lambda=\dfrac1v=4.34 \times 10^5cm$$
$$\lambda=4.34 \times 10^4nm$$
$$V=c(wavenumber)$$
$$V=3 \times 10^{10} cms^{-1}(23044.77cm^{-1})$$
$$=6.9 \times 10^{14}Hz$$
$$\lambda=4.34 \times 10^{-5} cm$$
Q11.28
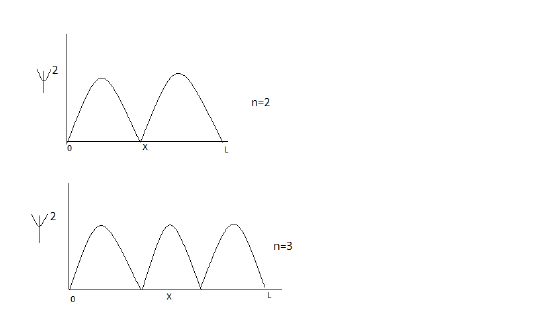
When an electronic transition is observed in a particle in a box model, where might the particle be when the energy level increases from level 2 (n=2) to level 3 (n=3). What is the relationship between nodes and energy level?
S11.28
To solve this question, graph $\psi^2$ for for both levels. $\psi^2$ is used because it tells us the probability of where a particle will be found.
The particle will be found in any region where $/psi$ is not equal to zero.
As energy level increases, the number of nodes increases.
Q12.21
Identify the second row homonuclear diatomic molecules that do not exhibit the normal characteristic of double bonds.
S12.21
O2 and F2 do not follow the normal characteristics of a double bond because they have the 2p σ and π bonding orbitals switched in terms of which is lower in energy.
Q14.3
Convert the following absorbance values to % transmittance.
a. 1.2, (b) .65, (c) 0.9
S14.3
To convert absorbance to % transmittance, use the formula below
a). A = 1.2
A = 2 - log (%T)
A + log (%T) = 2
log (%T) = 2 - 1.2 = 0.8
%T = 100.8
%T = 6.31
b). A = 0.65
A = 2 - log (%T)
A + log (%T) = 2
log (%T) = 2 - 0.65 = 1.35
%T = 101.35
%T = 22.39
c) A = 0.9
A = 2 - log (%T)
A + log (%T) = 2
log (%T) = 2 - 0.9 = 1.1
%T = 101.1
%T = 12.59
Q14.26
Determine the molecule with the highest fundamental frequency of vibration for the molecules given below.
B2, N2, O2
S14.26
The molecule with the smallest reduced mass gives the highest fundamental frequency.
$$ Reduced mass of B_2= \dfrac{(10.81amu)(10.81amu)}{10.81amu+10.81amu}=5.41amu$$
$$ Reduced mass of N_2=\dfrac{(14.007amu)(14.007)}{(14.007amu+ 14.007amu)}=7amu$$
$$ Reduced mass of O_2=\dfrac{(15.999amu)(15.999amu)}{15.999amu+15.999amu)}=8amu$$
Therefore, B2 has the highest fundamental vibration frequency.