Extra Credit 43
- Page ID
- 49204
Questions
Q2.74
The helium atoms that are sealed in a container all have the same initial speed with a temperature of the gas at equilibrium of 15K. The atoms are colliding with one another until the Maxwell distribution is reached. Assume no heat is exchanged between the gas and its surroundings. Find the initial speed of the helium atoms.
Relevant Information:
KB=1.381x10-23 J/K
Amu= 1.661x10-27 kg/amu
Q9.13
NO2 decomposes to 2NO and O2 in a second-order reaction. The initial pressure of NO2 is 3.20 atm at 670 °C and the half-life of the reaction is 2.55x103 min. Calculate the total gas pressure after one half-life and assume ideal gas conditions for all gases and that the volume remains constant.
Q10.3
The catalysis of dry sand is kickstarted by the enzyme hanvinase to turn it into gold. It will take the process 2 million years with the enzyme to cleave one dry sand molecule. Calculate the turnover rate of the enzyme.
Q11.3
Calculate the energy difference for the Bohr orbits from n=3 to 4 for atomic hydrogen.
Q11.27
Given the identity:
\(\int \Psi _{n}(x)\Psi _{m}(x)dx=\frac{2}{L} \int_{0}^{L}sin\frac{n\pi x}{L}sin\frac{m\pi x}{L}=0\)
What does this identity signify? Why is the concept expressed in this identity important for quantum chemistry?
Hint: In calculus, the integration of two multiplied vectors checks for orthogonality when a certain value is obtained.
Q12.20
Provide the total number of electrons, valance electron, electron configuration and bond order of carbonate ion, CO3-2. From the information describe the bonding.
Q14.2
Given the frequency, 7.7x1014 S-1, convert it into the wavelength and wavenumber.
Relevant Information:
c=3.00x108 m/s
Q14.25
Calculate the various degrees of freedom for the myoglobin molecule, which contain 9272 atoms.
Q2.52
The pressure of of a Neon atom hitting 5.0 cm2 of wall is 0.69 atm. Assuming the atom is traveling with a speed of 54,000 cm/s, find the force exerted by the Neon atom.
Q2.99
You are given 1 mole of O2(g) at 43ºC
Part I:
Match the following related speed expressions listed with the bank of values provided below.
| Speed Expression | Match these choices with the speed expression to the left. | |
| 1.) Vrms | a.) \(573\frac{m}{s}\) | d.) \(591\frac{m}{s}\) |
| 2.) Vmp | b.) \(469\frac{m}{s}\) | e.) \(702\frac{m}{s}\) |
| 3.) Vavg | c.) \(647\frac{m}{s}\) | f.) \(663\frac{m}{s}\) |
Part II:
Calculate the Z1 given that the collisional diameter of O2 is 0.361nm and the pressure is 1atm.
Answer Key
Q2.74: \(9.35x10^{4}cm/s\)
Q9.13: \(3.28atm\)
Q10.3: \(k=1.72x10^{-14}S^{-1}\)
Q11.3: \(\Delta E=1.059x10^{-19}\)
Q11.27: The identity indicates orthogonality. Orthogonality of wave functions is important in the structure of an atom and the relative locations of its electrons.
Q12.20: Valence electrons: 4+3(6)+2=24
Total number of electrons: 6+3(8)+2=32
Electron configuration: \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{10}4p^{2}\)
Bonding order: \(\frac{1}{2}(12-12)=0\)
Q14.2: \(\widetilde{\nu }=2.56x10^{6}m^{-1}\)
Q14.25:
| Nonlinear | 3 | 3 | 27810 | 27816Q2.52: |
Q2.52: \(Force=3.45x10^{-4}N\)
Q2.99: Part I: 1.) E 2.) A 3.) C
Part II: \(2.04\times 10^{-17}\frac{collisions}{s}\)
Solutions
S2.74
Because the energy is conserved, the total transitional energy of the atom can be reached after equilibrium is reached.
Suppose there are N helium atoms, the total energy is:
\(E_{trans}=N(\frac{1}{2}mv^{2})=N(E_{trans})\)
\(N(\frac{1}{2}mv^{2})=N(\frac{3}{2}K_{B}T)\)
Solve for speed (V):
\(V^{2}=\frac{3K_{B}T}{m}\)
\(V=\sqrt{}\frac{3K_{B}T}{m}\)
\(V=\sqrt{\frac{3(1.381x10^{-23}J/K)(15K)}{(4.003amu)(1.661x10^{-27}kg/amu)}}\)
V= 305.7 cm/s
S9.13
Given the chemical equation: \(2NO_{2}\rightarrow 2NO+O_{2}\)
We apply the ideal gas law \(PV=nRT\)
With constant volume and temperature, we expect a positive relationship between pressure and temperature. Therefore, we can make a rudimentary assumption on what we expect to happen just by analyzing this relationship.We assume that after one half life (the reduction of the reactant's concentration by one-half), we should expect a one-half decrease in pressure.
To prove this assumption, we apply the ideal gas law. \(PV=nRT\Rightarrow P=\frac{nRT}{V}\)
We can further simplify this equation.
Since we're only comparing between the pressures of reactants and products, we can omit the constant values of "R", "T" and "V" from our equation:
\(P=n\)
From there, we can derive avogadro's law: \(\frac{P_{1}}{n_{1}}=\frac{P_{2}}{n_{2}}\)
We can now calculate the product half-life pressures from this equation:
NO= \(\frac{3.20atm}{2mol}=\frac{P_{\frac{1}{2}NO}}{2mol}\) \(P_{\frac{1}{2}NO}= 1.6 atm\)
O2= \(\frac{3.20atm}{2mol}=\frac{P_{\frac{1}{2}O_{2}}}{1mol}\) \(P_{\frac{1}{2}O_{2}}= 0.8 atm\)
NO2= \(P_{\frac{1}{2}NO_{2}}= \frac{3.20atm}{2mol}= 1.6 atm\)
\(Total\, P_{\frac{1}{2}}=P_{\frac{1}{2}NO}+P_{\frac{1}{2}O_{2}}+P_{\frac{1}{2}NO_{2}}=1.6atm+0.8atm+1.6atm=3.28 atm\)
S10.3
Use the equation to find the Kcat:
\(t=\frac{1}{k}\)
Convert million into seconds:
\(2,000,000 year=\frac{12 month}{year}x\frac{4week}{month}x\frac{7day}{week}x\frac{24 hour}{day}x\frac{60 min}{hour}x\frac{60 sec}{min}=5.81x10^{13} sec\)
Find k from the equation by convert the equation:
\(k=\frac{1}{t}\)
\(k=\frac{1}{5.81x10^{13}S}=1.72x10^{-14}S^{-1}\)
S11.3
To calculate the energy difference from one energy state to another, we can use the Ryberg equation:
\(\Delta E=E_{f}-E_{i}=-R_{H}(\frac{1}{n^{2}_{f}}-\frac{1}{n^{2}_{i}})\)
Now plug in the initial and final state:
\(\Delta E=-2.179x10^{-18}(^{\frac{1}{4^{2}}}-^{\frac{1}{3^{2}}})\)
\(\Delta E=1.059x10^{-19}\)
S11.27
We know from calculus that the integration of the product of two vectors (or wavefunctions) will indicate that the 2 vectors (or wavefunctions) will be orthogonal to each other WHEN the integration yields an answer of zero.
Therefore, the answer to the first part of the question ("what does this identity signify?") is that the identity signifies that the two wave functions in question, \(\Psi _{n}(x)\: and\: \Psi _{m}(x)\), are orthogonal (perpendicular) with each other.
Why is orthogonality important?
It should be explained that the given identity, \(\int \Psi _{n}(x)\Psi _{m}(x)dx=\frac{2}{L} \int_{0}^{L}sin\frac{n\pi x}{L}sin\frac{m\pi x}{L}=0\), applies to wavefunctions of electrons in one atom.
Let's set some arbitrary meaning to our wave functions.
Let's say that \(\Psi _{n}(x)\) stands for the wavelength of a 2px electron, while \(\Psi _{m}(x)\) stands for the wavelength of a 2pz electron.
You want these orbitals to be orthogonal since you want these electrons to be accounted for in an atom. Imagine if these orbitals were not orthogonal, and were in a different orientation. Would it make sense?
The image below should provide a visual of orthogonal 2p orbitals. 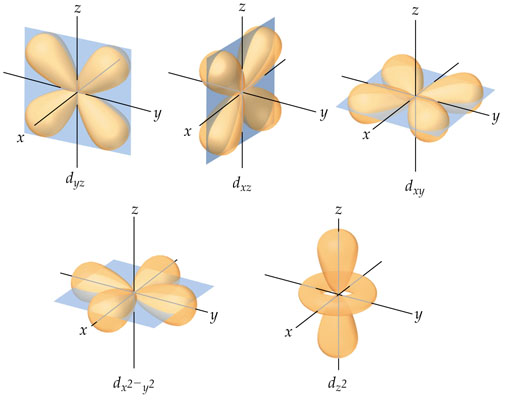
Image credit to: Chemistryland.com : http://www.chemistryland.com/CHM151S...tations512.jpg
S12.20
Valence electrons: 4+3(6)+2=24
Total number of electrons: 6+3(8)+2=32
Electron configuration: \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{10}4p^{2}\)
Bonding order: \(\frac{1}{2}(12-12)=0\)
S14.2
We have this equation:
\(\lambda =\frac{c}{\nu}=\frac{1}{\widetilde{\nu }}\)
First given with frequency and the constant of speed:
\(\lambda =\frac{3.00x10^{8}m/s}{7.7x10^{14}S^{-1}}=3.9x10^{-7}m\)
Use the newly found wavelength to find the wavenumber:
\(\widetilde{\nu }=\frac{1}{\lambda }=\frac{1}{3.9x10^{-7}m}=2.56x10^{6}m^{-1}\)
S14.25
Determine the molecule type: atoms, linear, and nonlinear
| Translation | Rotation | Vibration | Total | |
| Atoms | 3 | 0 | 0 | 3 |
| Linear | 3 | 2 | 3N-5 | 3N |
| Nonlinear | 3 | 3 | 3N-6 | 3N |
Myoglobin is a nonlinear molecule which has:
| Nonlinear | 3 | 3 | 27810 | 27816 |
S2.52
This is a trick question with a lot of useless information given. In order to solve it, you need to sort through useful from the useless.
To find the pressure exerted on the wall by the Neon atom:
\(Pressure=\frac{Force}{Area}\)
Only the given pressure and area is necessary:
\(0.69atm=\frac{Force}{((5.0cm^{2})(\frac{1m}{100cm})^{2})}\)
\(Force=3.45x10^{-4}N\)
S2.99
Part I:
Convert some of the given information to accommodate easier calculations.
\(43^{\circ}C +273K=316K\) \(Molar \: mass \: of\: O_{2} = 16\frac{g}{mol}\cdot \frac{1kg}{1000g}=.016\frac{kg}{mol}\)
\(8.314\frac{J}{molK}= 8.314\frac{m^{2}kg}{s^{2}molK}\)
There are three essential equations that we need to know to be able to do these calculations.
\(V_{rms}=\sqrt{\frac{3RT}{M}}\) \(V_{mp}=\sqrt{\frac{2RT}{M}}\) \(V_{avg}=\sqrt{\frac{8RT}{\pi M}}\)
Apply these equations to the given speed expressions
- \(V_{rms}=\sqrt{\frac{3RT}{M}}=\sqrt{\frac{3(.8.314\frac{m^{2}kg}{s^{2}molK})(316K)}{.016\frac{kg}{mol}}}=702\frac{m}{s}\) This answer matches with choice E.
- \(V_{mp}=\sqrt{\frac{2RT}{M}}=\sqrt{\frac{2(8.314\frac{m^{2}kg}{s^{2}molK})(316K)}{.016\frac{kg}{mol}}}=573\frac{m}{s}\) This answer matches with choice A.
- \(V_{avg}=\sqrt{\frac{8RT}{\pi M}}=\sqrt{\frac{8(8.314\frac{m^{2}kg}{s^{2}molK})(316K)}{\pi 0.016\frac{kg}{mol}}}=647\frac{m}{s}\) This answer matches with choice C.
Part II:
The equation for Z1 is:
\(Z_{1}=\sqrt{2}\pi d^{2}\overline{c}(\frac{n}{V}) \frac{collisions}{s}\)
Convert the collisional diameter from nanometers to meters.
\(.361nm\cdot (\frac{1\times 10^{-9}m}{1nm})=3.61\times 10^{-10}m\)
Substitute the expression for \(\frac {n}{V}\)
\(PV=nRT\) \(\frac{n}{V}=\frac{P}{RT}\)
Our new equation for Z1 is: \(Z_{1}=\sqrt{2}\pi d^{2}\overline{c}(\frac{P}{RT}) \frac{collisions}{s}\)
We plug our values into this new equation to give:
\(Z_{1}=\sqrt{2}\pi (3.61\times 10^{-10}m)^{2}(647\frac{m}{s})(\frac{1atm}{.08206\frac{atmL}{molK}(316K)}) =2.04\times 10^{-17}\frac{m^{3}}{Ls}\cdot (\frac{1L}{1m^{3}})=2.04\times 10^{-17}\frac{collisions}{s}\)

