Extra Credit 42.
- Page ID
- 49203
Questions
Q2.73
Fluorine (in the form of \(F_{2}\)) is a naturally occuring, pale-yellow diatomic gas with a molecular diameter of 1.417Å. Under “ultra-high-vacuum” experiments carried out at 300K, a mean free path of 4.97 x 10-5m is observed. What is the total pressure formed (in units of atm) under such conditions? Assume ideal gas conditions.
Relevant Information:
1Å \(=1.0\times 10^{-10}m\)
\(R=8.314 \tfrac{J}{molK} = .0821 \frac{L atm}{mol K}\)
\(K_{b}=1.38\times 10^{-23}JK\)
______________________________________________
Q9.12
You're working in a lab and you just found out that the reaction \(2P_{2}O\rightarrow 2P_{2}+O_{2}\) proceeds in the second-order. Answer the following questions about this reaction:
- How did you determine the rate order from the reaction formula?
- You want to know how much of the [P2O] is left after letting the reaction proceed for 6.95 seconds. Given that the rate constant is 0.45 M-1s-1 and we start with an initial concentration of [P2O]=0.82M, solve this problem. Also, what is the related half-life formula to this reaction?
_________________________________________________
Q10.3
a.)What is the difference between the pre-equilibrium enzyme-substrate binding scheme and the steady-state-scheme of enzyme-substrate binding?
b.) Consider this enzyme catalyzed reaction:
\(k_{1}=9.2\times 10^{8}M^{-1}s^{-1}\)
\(k_{-1}=10.6\times 10^{8}M^{-1}s^{-1}\)
\(k_{2}=8.3\times 10^{8}M^{-1}s^{-1}\)
Does the enzyme-substrate biding follow the steady-state scheme or the pre-equilibrium scheme? Briefly explain your answer?
If this reaction follows the pre-equilibrium scheme, calculate for the equilibrium constant K.
_________________________________________________
Q11.2
The threshold frequency for dislodging an electron from a cadmium metal surface is 9.865 x 1014Hz. Calculate both the wavelength (in nanometers) and energies required to remove an electron from the metal.
Hint: If a wave has more energy than the threshold energy, then electron is dislodged
Relevant Information:
Planck's constant ("h"): \(6.626\times 10^{-34}m^{2}kgs^{-1}\)
Speed of Light ("c"): \(3.0\times 10^{8}\frac{m}{s}\)
\(1 Hz = second^{-1}\)
_________________________________________________
Q11.26
1,3,5-hexatriene is a particle in a one-dimensional box with infinite walls potential. Calculate the change in energy associated with the electronic transition involved in the excitation of electrons from the highest filled level (n=4) to the lowest unfilled level orbital.
Relevant Information:
Bond length for a C-C single bond = \(154pm\)
Bond length for a C-C double bond = \(135pm\)
Distance equal to a C atom radius at each end = \(77pm\)
\(1pm=1\times 10^{-12}m\)
\(m_{e}=9.1095\times 10^{-31}kg\)
Planck's constant ("h"): \(6.626\times 10^{-34}m^{2}kgs^{-1}\)
_________________________________________________
Q12.19
Cyanide (CN1-) is a fast-acting and fatal chemical compound that exists in both the gas and salt phase. Answer the following questions about Cyanide.
- How many total electrons does it have? Of those electrons, how many are valence electrons?
- Write down its electron configuration.
- What is its bond order? In terms of molecular orbital theory, describe the bonding scheme of the CN1- compound.
- Compare the bond order of CN1- with a carbide ion (C22-).
_________________________________________________
Q14.1
You have a report due in 30 minutes and you've just accidentally broken your lab's Infrared Spectrometer. Luckily for you, the lazy intern, Antonio, will have his turn with the IR spectrometer in 30 minutes. You decide to sabotage him and blame him for the broken machine in order to retain your job. To successfully do this, you must present your report with accurate results from the IR spectra. After a couple of trial runs, you somehow realize that the machine gives you an accurate reading for the wavelength but not the frequency and wavenumber. Your project's chemical compound in focus,"II-infinityandbeyonceoate", produces a wavelength of 469nm. What is it's frequency (s-1) and wavenumber (cm-1)?
_________________________________________________
Q14.24
For each given molecule give the following information:
a) Name the geometry of the molecule
b) 2 possible vibration modes it may have
c) Pick one vibration mode and indicate whether it is IR active or not.
- \(H_{2}O\)
- \(CO_{2}\)
_________________________________________________
Q2.50
- Choose the correct answer that makes the statement correct: Temperature, volume and pressure are all classified as (microscopic or macroscopic) concepts.
- From your choice above, explain why such concepts are imperative to thermodynamics and the study of gases. Assume ideal gas conditions.
_________________________________________________
Q2.95
An industrial chemical plant explodes in Gatorville, Florida. Above this wreck, a new species of bird is flying 1 meter above the thermosphere (T=240K) and dies midair due to poisoning from the exuded fluorine (F2) and chlorine (Cl2) gases. It is known that only one of these gases will be more able to escape the thermosphere than the other. Which of these two molecules will have a greater tendency to escape?
Relevant information:
\(Earth's\; gravitational\; field\;= (2\frac{GM}{r} )^{\frac{1}{2}}\)
\(G=universal\: gravitational\: constant=6.67\times 10^{-11}m^{3}kg^{-1}s^{-2} \)
\(M=mass\:of\:Earth=6.0\times 10^{24}kg \)
r= distance from the center of the Earth to the object
\(Boltzmann's\: constant= K_{B}=1.381\times 10^{-23}\frac{J}{K}\)
Answer Key
A2.73: 9.22 atm
A9.12: 1.)You can't. 2.) 0.230M
A10.3: a.) see S10.3 b.) Pre-Equilibrium
A11.2: 304nm
A11.26: \(4.01\times 10^{-19}J\)
A12.19:
- Total electrons = 14 ; Valence electrons = 10
- \((\sigma _{1s})^{2}(\sigma _{1s}^{\ast })^{2}(\sigma _{2s})^{2}(\sigma _{2s}^{\ast })^{2}(\sigma _{2p})^{2}(\pi _{2p})^{4} \)
- Bond Order = 3 ; Bonding Scheme = triple bond (1 sigma bond, 2 pi-bonds)
- Carbide ion has 14 total electrons and 10 valence electrons. Bond Order = 3 with exactly the same electron configuration as CN1-
A14.1: \(f=6.40\times 10^{14}s^{-1}\; ;\; \widetilde{\nu }=21,322cm^{-1}\)
A14.24:
- a.) Bent/Angular b.) Pick 2: Symmetric stretch/Antisymmetric stretch/Scissoring bend. c.) Pick 1 vibration mode; All forms are Infrared Active.
- a.) linear b.)Symmetric stretch/Antisymmetric stretch/ Degenerate c.) Pick 1 vibration mode: Symmetric stretch: IR inactive/ Antisymmetric Stretch and Degenerate are both IR active.
A2.50: 1.) macroscopic 2.) see S2.50 below
A2.95: F2
Solutions
S2.73
The mean free path is the average distance a gas particle travels between collisions with other particles. With this in mind, we can somewhat predict what our answer for pressure look like. With a small mean free path of \(1.417\times 10^{-10}m\), we expect our total pressure to be high, in order to account for the small distance between particle collisions.
One equation that gives us the mean free path is:
\(\lambda =\frac{K_{B}T}{\sqrt{2}P\pi d^{2}}\)
It would be best if we convert the molecular diameter from Angstroms to meters:
\(1.417 Angstroms\cdot (\frac{1.0\times 10^{-10}m}{1 Angstrom})=1.417\times 10^{-10}m\)
Plugging our given values, we get the expression:
\(\frac{1.38\times 10^{-23}\frac{J}{K}(300K))}{\sqrt{2}P\pi (1.417\times 10^{-10}m)^{2}}=4.97\times 10^{-5}m\)
We then solve for our unknown (P)
\(\frac{\sqrt{2}P\pi (1.417\times 10^{10}m)^{2}}{1.38\times 10^{-23}\frac{J}{K}(300K)}=\frac{1}{4.97\times 10^{5}m}\)
\(P=933.8\frac{J}{m^{3}}\cdot (\frac{.0821\frac{Latm}{molK}}{8.314\frac{J}{molK}})(\frac{1m^{3}}{1L})=9.22atm\)
S9.12
1.) You can't. Rate laws are experimentally determined and can't be derived from the chemical equation. When determining the rate laws, always ask: SHOW ME THE DATA.
2.) There are different rate laws for different order reactions. Since we know the reaction occurs in the 2nd-Order (determined experimentally), then we apply the second order rate law to calculate for our unknown.
Use the rate law for a second order reaction and calculate for the unknown ([A]).
\(t_{\frac{1}{2}}=\frac{1}{k\left [ A \right ]_{0}}\)
\(\frac{1}{[A]}=kt+\frac{1}{[A]_{0}}=0.45M^{-1}s^{-1}(6.95s)+\frac{1}{0.82M}=4.35M^{-1}\)
\([A]=\frac{1}{4.35M^{-1}}=.230M\)
The answer checks out since we expect our initial [A] to be higher than the final [A] since more of the reactants are converted into products.
S10.3
\(A\rightleftharpoons B\rightarrow C\)
a.)These two assumptions are separated in which:
- The steady-state assumption assumes a rate of change of 0 for the formation of an intermediate "B".
- The pre-equilibrium assumption assumes a state of "pre-equilibrium" between the initial reactant and the formed intermediate. In this assumption, the rate of formation of the intermediate is slow. This means that the rate for k-1 (the reverse reaction) is greater than the forward reaction that allows for the formation of the intermediate (k1).
b.) From the answer provided in part (a), it is clear to see that the given reaction follows the pre-equilibrium scheme more than the steady-state scheme.
\(K=\frac{K_{1}}{K_{-1}}\)
\(K=\frac{9.2x10^{8}M_{-1}S_{-1}}{10.6x10^{8}M_{-1}S_{-1} }=0.87\)
S11.2
Calculate the amount of energy the threshold barrier has:
\(E=h\upsilon=6.626\times 10^{-34}m^{2}kgs^{-1}(9.865\times 10^{14}Hz)=6.54\times 10^{-19}J\)
Calculate the wavelength associated with the previously calculated energy
\(E=\frac{hc}{\lambda }=\frac{6.626\times 10^{-34}Js(3.0\times 10^{8}\frac{m}{s})}{\lambda }= 6.54\times 10^{-19}J \)
\(\lambda =3.04\times 10^{-7}=304nm\)
We want the energy of a wave to be greater than the threshold energy of 6.54x10-19J. Shorter wavelengths than 304 nm are therefore required to obtain higher energies and thus overcome the threshold barrier allowing for electron dislodging.
S11.26
For Polyenes, the equation for energy transition is as follows: \(\Delta E=(N+1))\frac{h^{2}}{8m_{e}L^{2}}\)
We must calculate for the value of L, the length of the molecule:
- 2 single carbon bonds= \(2\times 154pm=308pm\)
- 3 carbon double bonds= \(3\times 135pm=405pm\)
- 2 C atom radii (from each end of the molecule)= \(2\times 77pm=154pm\)
Total= \(308pm+405pm+154pm=867pm\cdot (\frac{1m}{1\times 10^{12}pm})=8.67\times 10^{-10}m\)
Plug in the calculated value for "L" into the polyene energy transition equation:
\(\Delta E=(4+1)\frac{(6.626\times 10^{-34}m^{2}kgs^{-1})^{2}}{8(9.1095\times 10^{31}kg)(8.67\times 10^{-10}m)^{2}}=(5)\frac{4.39\times 10^{-67}m^{4}kg^{2}s^{-2}}{8(9.1095\times 10^{31}kg)(7.52\times 10^{-19}m^{2})}=4.01\times 10^{-19}m^{2}kgs^{-2}=4.01\times 10^{-19}J\)
S12.19
- 14 total electrons. Of those 14, 10 are valence electrons. Valence electrons are counted from the highest energy subshells of an atom. Carbon has 2 electrons from the 2s subshell, and 2 electrons from the 2p subshell. Nitrogen has 2 electrons from the 2s subshell and 3 electrons from the 2p subshell. An additional electron also resides in the 2p subshell between the two atoms, contributing to the molecule's overall -1 charge. In total, the number of valence electrons amount to: \(2+2+2+3+1=10\) valence electrons
- Tip: A molecular orbital diagram would be helpful when constructing the molecular electron configuration.
To obtain the molecular electron configuration, we always begin with the lowest energy orbital and work our way to the highest possible orbital the compound's electrons occupy. We include both bonding and antibonding orbitals in the electron configuration in order to account for all electrons present in the molecule.
Additionally, the format of the electron configuration is as follows: ("bond type"electron subshell)number of electrons
The electron configuration stops when you account for the last electron in the highest possible orbital.
\((\sigma _{1s})^{2}(\sigma _{1s}^{\ast })^{2}(\sigma _{2s})^{2}(\sigma _{2s}^{\ast })^{2}(\sigma _{2p})^{2}(\pi _{2p})^{4} \)
As an added precaution, it might be helpful to count all the electrons in your written electron configuration:
\(2(\sigma 1s)+2(\sigma 1s*)+2(\sigma 2s)+2(\sigma 2s*)+2(\sigma2p)+4(\pi2p)=14\) total electrons - \(Bond\; Order =\frac{bonding\; electrons-antibonding\; electrons}{2}\)
From the electron configuration above:
# Bonding electrons = \(2+2+2+4=10\) #Antibonding electrons (denoted by asterisk (*)= \(2+2=4\)
\(Bond\; Order =\frac{10-4}{2}=3\)
A bond order of 3 denotes a triple bond between the two atoms in the compound. This is further supported by the fully filled \(\mathbf{\sigma _{2p}}\) and \(\mathbf{\pi _{2p}}\) orbitals, and their empty, antibonding counterparts. The filled \(\mathbf{\sigma _{2p}}\) orbital signifies the sigma bond, and the 4 electrons from the \(\mathbf{\pi _{2p}}\) orbitals signify the 2 pi-bonds in a triple bond. - A carbide ion has 14 total electrons and 10 valence electrons. Its electron configuration appears exactly as a cyanide molecule's. As such, the bond order of a carbide ion should be the same as cyanide's. The bond order should equal to three, and the two carbon atoms should be bound by one sigma bond and two pi-bonds, thus forming a triple bond.
S14.1
To calculate for the frequency: \(frequency=f=\frac{c}{\lambda }\)
\(f=\frac{3.0\times 10^{8}\frac{m}{s}}{469nm\cdot (\frac{1m}{1\times 10^{9}nm})}=6.40\times 10^{14}s^{-1}\)
To calculate for the wavenumber: \(Wavenumber=\widetilde{\nu }=\frac{1}{\lambda }\)
\(\widetilde{\nu }=\frac{1}{469nm}\cdot (\frac{1\times 10^{7}nm}{1cm})=21,322cm^{-1}\)
To absolve yourself from your sins after sabotaging an innocent intern: \(Repentance=Ask\;God \;for \;forgiveness\)
S14.24
Overview of IR Activity: In order for a spectroscopic transition (which indicate Infrared Activity) to occur, the molecule's dipole moment must change during a vibration. This is why homonuclear molecules (like \(O_{2},F_{2}\) and \(P_{4} \)) are IR inactive.
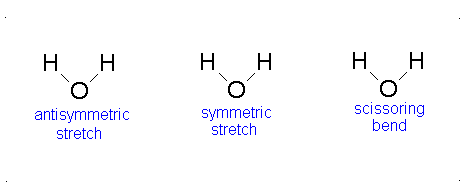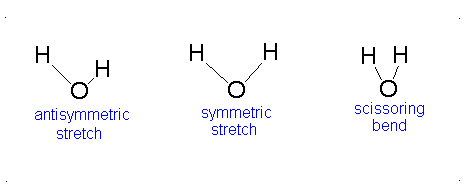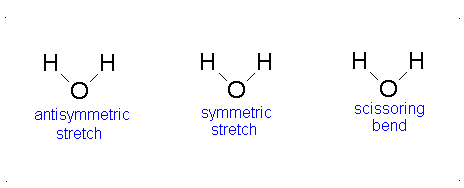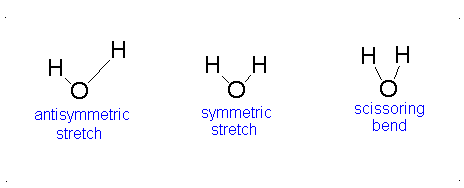
a.) Bent or Angular
b.) Pick 2 of the following:antisymmetric stretch, symmetric stretch, scissoring bend.
c.) Since all vibrational modes of \(H_{2}O\) involve changes in dipole moments, all vibrational modes of \(H_{2}O\) are IR active.-
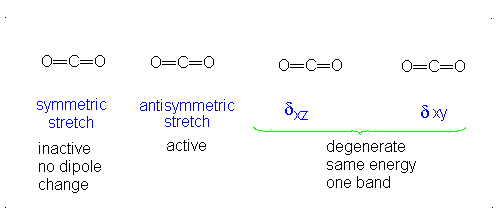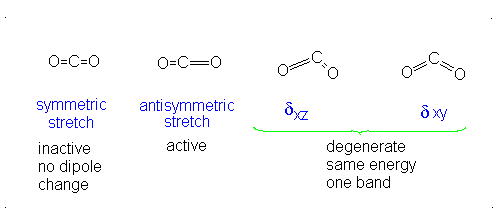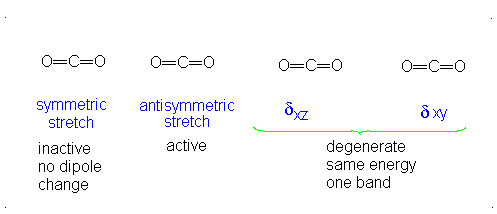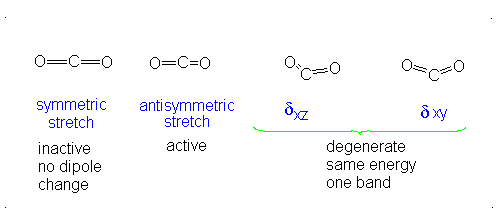
a.) Linear
b.) Pick 2 of the following: symmetric stretch, antisymmetric stretch, degenerate
c.) Dipole moment changes are observed in the antisymmetric stretch and degenerate vibration forms. Both of these forms are IR active. Since no dipole moment is generated in a symmetric stretch, this vibration mode is IR inactive.
S2.50
- Answering this question requires an understanding of both microscopic and macroscopic concepts.
Microscopic concepts: These concepts mainly look at activity in the molecular level.
Macroscopic concepts: These concepts look at the overall collective activity of microscopic systems (atoms, molecules, photons etc...)
The activity of temperature, volume and pressure are not constrained to the activity of just one system, but a collection of microscopic systems. Therefore, these constituents are thought to be macroscopic concepts.
*** It is also important to distinguish between heat and temperature. Heat in itself is a microscopic concept since it only depends on the movement of molecules, whereas apparent temperatures depend on more variables. - One of Thermodynamics' main points is that it measures the macroscopic quantities and ignores the microscopic activities of individual molecules. In short, Thermodynamics provides a framework for relating different macroscopic concepts with each other.
To enhance your understanding, consider this analogy. The efficiency of a refrigerator is measured through many different aspects of the machine, not just the type of liquid coolant it has.
S2.95
This is a trick question that gives too much information. The equation that relates velocity from Earth's gravitational field will yield the same velocity since the values for "G", "M", and "r" do not change in between the two given gases. This equation is therefore, essentially useless.
Think of the tendency for a gas to escape. We know that gases with higher pressures will have greater tendencies to escape. The more often gas molecules collide, the higher the chance for this gas to find a way out and escape.
With this known, the amount and frequency of molecular collisions depend on the speed of a molecule. The faster a molecule moves, the more frequently it will collide.
The question therefore shifts to which molecule travels faster? F2 or Cl2?
To solve this question, we must apply our knowledge of Kinetic Energies.
The average translational kinetic energy of any kind of molecule in an ideal gas is given by:
\(KE=\frac{3}{2}K_{B}T\)
We also know that kinetic energy can also equal:
\(KE=\frac{1}{2}mv^{2}\)
Setting these two equations equal to each other, as well as plugging in our known values, we get these expressions:
F2: \(\frac{3}{2}1.381\times 10^{-23}\frac{J}{K}(240K)=\frac{1}{2}(.019kg)v^{2}\) \(v=7.234\times 10^{-10}\frac{m}{s}\)
Cl2: \(\frac{3}{2}1.381\times 10^{-23}\frac{J}{K}(240K)=\frac{1}{2}(.0355kg)v^{2}\) \(v=5.292\times 10^{-10}\frac{m}{s}\)
F2 has a faster velocity (which makes sense since Cl2 molecule is heavier). F2 therefore has a greater tendency to escape.

