Extra Credit 39
- Page ID
- 49199
Q2.70
Choose the best statement that best describes the mean free path
- the path a molecule that uses free energy
- average distance traveled by a moving molecule between collisions.
- the volume of a gas is directly related to the number of moles of atoms contained in the gas
- values for temperature and volume of a gas are directly related
S2.70
Answer (b) The mean free path is the average distance a particle travels before a collision with another particle.
The equation used to solve for the mean free path is:
\[\lambda = \dfrac{1}{\sqrt{2}\pi(d^2)(\dfrac{N}{V})}\]
- \(\lambda\) is the mean free path.
- \(d\) is the diameter of the particle's collisional cross-section.
- \(d\) is inversely proportional to \(\lambda\), so increasing \(d\) will decrease \(\lambda\) and decreasing \(d\) will increase \(\lambda\)
- \(\dfrac{N}{V}\) is the particle density of the corresponding system, where \(N\) is the number of particles in the system and \(V\) is the volume of the the system.
- \(\dfrac{N}{V}\) is inversely proportional to \(\lambda\), so increasing \(\dfrac{N}{V}\) will decrease \(\lambda\) and decreasing \(\dfrac{N}{V}\) will increase \(\lambda\)
- \(N\) is inversely proportional to \(\lambda\), so increasing \(N\) will decrease \(\lambda\) and decreasing \(N\) will increase \(\lambda\)
- \(V\) is directly proportional to \(\lambda\), so increasing \(V\) will increase \(\lambda\) and decreasing \(V\) will decrease \(\lambda\)
Q2.80
If equal amounts of \(Kr\) and \(Xe\) are placed in a porous container and allowed to escape, which gas will escape faster and how much faster? Why? Assume room temperature and the pressure is 1.00 atm.
S2.80
For this problem, we have to use Graham's Law to help us solve this.
Helpful information:
-
Graham's Law: \(\dfrac{r_1}{r_2} = \sqrt{\dfrac{M_2}{M_1}}\)
-
\(Kr\)'s molecular weight: \(83.798\dfrac{g}{mol}\)
-
\(Xe\)'s molecular weight: \(131.293\dfrac{g}{mol}\)
We can assign \(Kr\)'s molecular weight to \(M_1\) and \(Xe\)'s molecular weight to \(M_2\).
So: \(\dfrac{r_1}{r_2} = \sqrt{\dfrac{M_2}{M_1}} = \sqrt{\dfrac{131.293\dfrac{g}{mol}}{83.798\dfrac{g}{mol}}} = 1.25\)
Therefore, \(Kr\) will escape \(1.25x\) faster than \(Xe\). Intuitively, this makes sense because \(Kr\) is a smaller molecule than \(Xe\), so it has a greater probability of escaping the porous container.
Q9.9
Nitrosyl Chloride was used as a key ingredient in aqua regia. This compounds dissolves precious metals such as gold and platinum, and it will also dissociate into nitric oxide gas and chlorine gas.
Given the reaction:
\[NOCl(g) → NO(g) + Cl_2(g)\]
and the data:
| [NOCl]0(M) | 0.1 | 0.2 | 0.3 |
| Rate(mol/L/h) | 3.7x10-8 | 1.48x10-7 | 3.33x10-7 |
Determine the rate equation, the rate constant, and the overall order of the reaction.
S9.9
First, we have to balance the reaction:
\[2 NOCl(g) → 2 NO(g) + Cl_2(g)\]
From this, we can determine the general rate law:
\[r = k[NOCl]^n\]
If we compare the initial rates between the first two experiments, we can see that by doubling \([NOCl]_0(M)\) from \(0.1\) to \(0.2\), the initial rate will quadruple from \(3.7x10^{-8}\) to \(1.48x10^{-7}\). This means that \(n = 2\) and the reaction is 2nd order.
Since: \(r = k[NOCl]^n\)
That means: \(k = \dfrac{r}{[NOCl]^2}\)
Now, we can take the data from any one of the experiments and input it into the equation to find the rate constant.
Using the first experiment: \(k = \dfrac{r}{[NOCl]^2} = \dfrac{3.7x10^{-8}}{0.1^2} = 3.7x10^{-6} L/mol/s\)
Q9.17
Define half-life. Then for 0th order, 1st order, and 2nd order reactions, give their equations that solve for half-life and their dependence on the original concentration.
S9.17
Half-life is the time it takes for the concentration of the reactant to decrease to half its original value.
| Order | Equation | Dependence |
| 0th order | \(t_{1/2} = \dfrac{[A]_0}{2k}\) | dependent |
| 1st order | \(t_{1/2} = \dfrac{ln2}{k} = \dfrac{0.693}{k}\) | independent |
| 2nd order | \(t_{1/2} = \dfrac{1}{k[A]_0}\) | dependent |
Q9.33
Given the data:
| Temperature (K) | k (M-1min-1) |
| 297 | 1.7 |
| 314 | 2.4 |
| 411 | 37 |
| 520 | 53 |
Determine the activation energy for this reaction.
S9.33
For this problem, we have to use the Arrhenius Equation, which is:
\(k = Ae^{-\dfrac{E_A}{RT}}\)
Taking the natural log of the equation, we get:
\(ln(k) = ln(A) - \dfrac{E_A}{RT}\)
To find the activation energy of the reaction, we have to compare two different sets of data at two different temperatures.
We now have 2 separate Arrhenius equations to deal with:
\(ln(k_1) = ln(A) - \dfrac{E_A}{RT_1}\) & \(ln(k_2) = ln(A) - \dfrac{E_A}{RT_2}\)
And when we subtract one from the other, we get:
\(ln(\dfrac{k_2}{k_1}) = \dfrac{-E_A}{R}(\dfrac{1}{T_2} - \dfrac{1}{T_1})\)
Using the first and last sets of data, we can now assign variables.
- \(T_1\) = 297K
- \(T_2\) = 520K
- \(k_1\) = 1.7M-1min-1
- \(k_2\) = 53M-1min-1
So: \(ln(\dfrac{53M^{-1}min^{-1}}{1.7M^{-1}min^{-1}}) = \dfrac{-E_A}{8.314Jmol^{-1}K^{-1}}(\dfrac{1}{520K} - \dfrac{1}{297K})\)
And solving for \(E_A\), we get: \(E_A = 19.805 kJ\)
Q10.8
Cyclohexane converts between the chair and boat conformations with the enthalpy of the formation of the activated complex to be 73.8 kJ mol-1 and the entropy of the formation of the activated complex to be 43.4 J mol-1 K-1. Determine the Gibbs energy of activation and the rate constant at room temperature.
S10.8
Useful equations
- Gibbs Free Energy: \(ΔG°^‡ = ΔH°^‡ - TΔS°^‡\)
- Transition State Theory \(k = \dfrac{k_BT}{h}e^{\dfrac{ΔS°^‡}{R}}e^{\dfrac{-ΔH°^‡}{RT}}(M^{1-m}) = \dfrac{k_BT}{h}e^{\dfrac{-ΔG°^‡}{RT}}(M^{1-m})\)
First, we use the Gibbs Free Energy equation to find \(ΔG°^‡\)
So: \(ΔG°^‡ = ΔH°^‡ - TΔS°^‡ = 73.8 kJ mol^{-1} - (297 K)(43.4 J mol^{-1} K^{-1})(\dfrac{1kJ}{1000J}) = 60.91 kJ mol^{-1}\)
Next, we can either use the Transition State Theory equation with the \(ΔG°^‡\) term or with the \(ΔH°^‡\) and \(ΔS°^‡\) terms. Let's use the equation with the \(ΔH°^‡\) and \(ΔS°^‡\) terms. Also, since cyclohexane performs its conversion by itself, this is a unimolecular reaction.
\(k = \dfrac{k_BT}{h}e^{\dfrac{ΔS°^‡}{R}}e^{\dfrac{-ΔH°^‡}{RT}}(M^{1-m}) = \dfrac{1.38x10^{-23} J K^{-1} * 297 K}{6.626x10^{-34} Js}e^{\dfrac{43.4 J mol^{-1} K^{-1}}{8.314Jmol^{-1}K^{-1}}}e^{\dfrac{-73.8 kJ mol^{-1} * 1000 \dfrac{J}{kJ}}{8.314Jmol^{-1}K^{-1} * 297 K}}(M^{1-1})\)
\(k = 119.8 s^{-1}\)
Q11.23
For a particle in a box of length L, normalize the ground-state wavefunction.
S11.23
When we substitute \(E_n = \dfrac{n^2h^2}{8mL^2}\) into \(Ψ = Asin(\dfrac{8\pi^2mE}{h^2})^{1/2}x\), we get \(Ψ_n = Asin\dfrac{n\pi}{L}x\).
To normalize the ground-state wavefunction, we start with the knowledge that the particle cannot leave the box. This means that the total probability to find the particle inside the box must be 1 or 100%. Knowing this, we can now integrate the wave function equation.
This becomes: \(Ψ^2 = A^2\int_{0}^{L}sin^2(\dfrac{n\pi}{L}x) = 1\)
\(A = \sqrt{\dfrac{2}{L}}\)
Q12.16
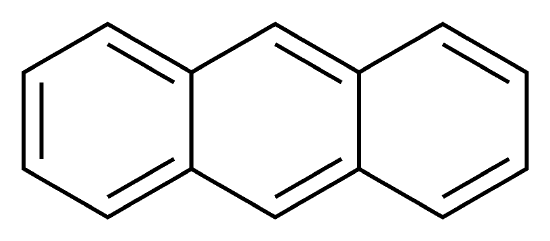
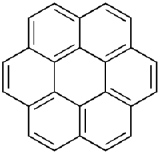
Anthracene is a solid polycyclic aromatic hydrocarbon, and is made up of three fused benzene rings. It is used in the production of the red dye alizarin and other dyes. Coronene is made up of 6 fused benzene rings. It is used as a solvent probe, similar to pyrene.
Between anthracene and coronene, which molecule has a greater degree of \(\pi\)-electron delocalization?
Conjugated polyenes are important in biological systems for several reasons. Give one example of polyenes, besides these two, that is used in a conjugated system and give it's biological system.
(anthracene) (coronene)
S12.16
- Coronene has a larger conjugated system with three more rings than naphthalene, therefore it has a greater degree of \(\pi\)-electron delocalization.
- Rhodopsin is a biological pigment found in the rods of the retina. It is extremely sensitive to light, and thus enables vision in low-light conditions. When exposed to light, it immediately photobleaches.
Q13.18
Which of the molecule's isomers has a higher melting point? Why?
S13.18
The para isomer has a higher melting point than the ortho isomer. Generally speaking, para isomers are more symmetrical in terms of their structure when compared to their ortho isomer counterparts. This allows their molecules to pack more closely together more easily and create a greater number of intermolecular bonds. As a result, forces of attraction are stronger, and therefore, a greater amount of energy would be required to break its bonds, so it would melt at a higher temperature.
Q14.21
In spectroscopy, there is a phenomenon known as the Doppler effect. Explain what this is in terms of light, then explain how this relates to sound and give a real world example of this.
S14.21
In general, the Doppler effect is the change in frequency of a wave for an observer that's moving relative to its source. In terms of visible light, the Doppler effect is observed when light becomes blue-shifted (light waves moving relatively faster to its receiver, increasing its apparent frequency) or red-shifted (light waves moving relatively slower to its receiver, decreasing its apparent frequency). In terms of sound, the Doppler effect is observed when the pitch gets higher (sound waves moving relatively faster to its receiver, increasing its apparent frequency) or gets lower (sound waves moving relatively slower to its receiver, decreasing its apparent frequency). An example of the Doppler effect for sound is when a moving car drives past you. The pitch is higher when the car is moving towards you, but gets lower when the car passes you.
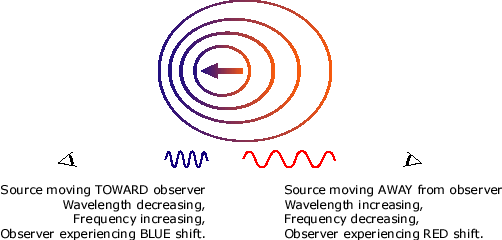
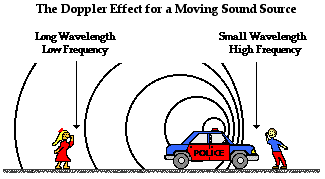
(light) (sound)