Extra Credit 32
- Page ID
- 49192
Q2.58
At what temperature will Li atoms have the same crms value as N2 molecules at 25°C? Solve this problem without calculating the value of crms for N2.
Relevant Information:
- Molar Mass of Li: ML = 6.94 g mol-1
- Molar Mass of N2: MN = 28.014 g mol-1
Q9.2
The rate law for the reaction
NH4+ (aq) + NO2- (aq) → N2 (g) + 2H2O (l)
is given by rate = k[NH4+][NO2-]. At 25°C, the rate constant is 3.0 x 10-4M-1s-1. Calculate the rate of the reaction at this temperature if [NH4+] = 0.38 M and [NO2-] = 0.058 M.
Q9.26
Use equation 9.23,
k = A e-Ea/RT
to calculate the rate constant at 350 K for Ea = 0, 2, and 50 kJ mol-1. Assume that A = 1010 s-1 in each case.
Relevant Information:
- Gas Constant: R = 8.3145 J mol-1 K-1
Q10.1
Does a catalyst affect the rate of a reaction in both directions? How? Explain your reasoning.
Q11.16
The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17J. For light that is 450 nm in wavelength, how many photons does this correspond to?
Relevant Information:
- Planck's constant: h = 6.626 x 10-34 J s
- Speed of Light: c = 3.00 x 108 m s-1
Q12.7
Consider the following Lewis structure for aluminum trifluoride (AlF3):
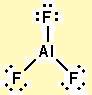
Does it satisfy the octet rule? If not, draw additional resonance structures that do satisfy the octet rule.
Q13.11
Calculate the bond enthalpy of NaF using the Born-Haber cycle. The bond length of NaF is 1.93 Å. See Tables 11.4 and 11.5 for other information. Use n=10 in Equation 13.7.
Q14.14
What is the degeneracy of the rotational energy level with J = 10 for a diatomic rigid rotor?
[The degeneracy is given by (2J +1).]
Q2.70
How does the crms of a gas depend on (a) the temperature at constant volume, (b) the density, (c) the pressure at constant temperature, (d) the volume at constant temperature, and (e) the size of molecules?
Q9.9
The progress of a reaction in the aqueous phase was monitored by the absorbance of a reactant at various times:
Time/s 0 54 171 390 720 1010 1190
Absorbance 1.67 1.51 1.24 0.847 0.478 0.301 0.216
Plot A (absorbance) vs t graph and determine the rate constant (k). Show how you find k from the graph.
Answer Key
Q2.58: 73.82 K
Q9.2: 6.6 x 10-6 M s-1
Q9.26:
For Ea = 0 kJ mol-1,
k = 1010 s-1
For Ea = 2 kJ mol-1,
k = 5.0 x 109 s-1
For Ea = 50 kJ mol-1,
k = 3.45 x 102 s-1
Q10.1: A catalyst affects the rate of a reaction in both directions by lowering the activation energy.
Q11.16: 91 photons
Q12.7: It does not satisfy octet rule.
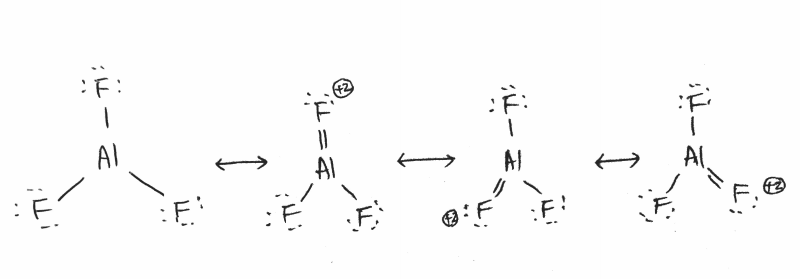
While these new structures do follow the octet rule with respect to Al, it is important to remember that aluminum is often an exception to the octet rule, as it can form stable compounds with only three bonds. Because of this, aluminum is commonly said to follow the sextet rule. Aluminum is following the sextet rule in the first Lewis structure. Compounds with aluminum can be stable following either the sextet or the octet rule.
Q13.11: -648 kJ mol-1
Q14.14: 21
Q2.70:
(a) The crms is dependent of temperature at constant volume.
(b) The density does not affect the crms.
(c) The crms is independent of the pressure at constant temperature.
(d) As the volume changes at constant temperature, as like the pressure above, there is no affect on crms.
(e) The size of the molecules does not affect the crms.
Q9.9:
Contextualizing the data in all three integrated rate law forms (i.e. 0th, 1st and 2nd), we find that the data is first order.
k = .0017 s-1. The slope of the line is -k.
Solutions
S2.58
crms is defined as
$$\sqrt{\frac{3RT}{M}}$$
Since crms of Li and crms of N2 should be the same,
$$\sqrt{\frac{3RT_L}{M_L}}$$
should be the same with
$$\sqrt{\frac{3RT_N}{M_N}}$$
So, If you set both of the equations equal to each other, you can square both sides. That gets rid of the square root. Next, we can easily cancel out the 3R from both sides as well. Therefore,
$$\frac{T_L}{M_L}$$
is equal to
$$\frac{T_N}{M_N}$$
TLi is now equal to
$$\frac{(M_L)(T_N)}{M_N}$$
Now, first we need to convert the temperature from Celsius to Kelvin.
T (in °C) + 273 = T (in K)
25 + 273 = 298 K
Plugging it in, we get
$$\frac{(6.941 g/mol)(298K)}{28.02 g/mol}$$
= 73.82 K
- R is gas constant.
- M is the molar mass in $ kg mol^{-1} $. In this case, we can treat M as the regular $ g mol^{-1} $ since we are working with a ratio of two different chemical species.
S9.2
In this question, we use Rate = k [NH4+][NO2-] to calculate the rate of the reaction.
The rate constant and concentration of reactants are given, so we can just plug the values into the equation to get the rate.
Rate = k [NH4+][NO2-] = (3.0 x 10-4 M-1s-1)(0.38M)(0.058M) = 6.6 x 10-6 M s-1
S9.26
We use The Arrhenius Equation that describes the temperature dependence of many reactions.
k = A e-Ea/RT
For Ea = 0 kJ mol-1,
\[k = (10^{10} s^{-1}) e^{\dfrac{-0 J mol^{-1}}{(8.314 J mol^{-1} K^{-1}) (350 K)}} = 1010s-1\]
For Ea = 2 kJ mol-1,
\( k = (10^{10} s^{-1}) e^{\frac{-2000 \frac{J}{mol}}{8.314 \frac{J}{mol \cdot K} 350K} \) = 5.0 x 109 s-1
For Ea = 50 kJ mol-1,
\( k = (10^{10} s^{-1}) e^{\frac{-50000 \frac{J}{mol}}{8.314 \frac{J}{mol \cdot K} 350K} = 3.45 x 102 s-1
Note: Remember to convert from kJ to J (because you want the units to cancel out and the gas constant is J mol-1 K-1
S10.1
A catalyst affects the rate of a reaction in both directions by lowering the activation energy, which is the energy hill between the reactants and the products of the reaction. Since small activation energies allows both reactants and products to overcome the barrier easier than before, the rate of a reaction in both directions should be faster than before (without catalyst). Here is a picture of what the effect of a catalyst looks like:
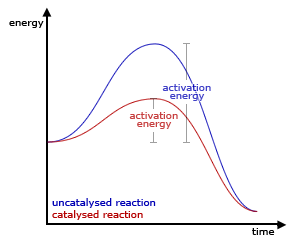
*Note: This is the picture was taken from this website for educational purposes from this website
http://ch302.cm.utexas.edu/kinetics/...alysts-all.php
As you can see when you compare the red and blue lines, the lowering of the energy barrier will have an effect on both the progress from reactants to products and products to reactants. However, this is an oversimplification of how a catalyst works. In reality, the catalysts works through multiple intermediates with a lower activation energy to get to the products (or the other way around).
S11.16
Since the question is asking to calculate the number of photons, we need to calculate how much energy a single 450 nm photon has first.
The energy of a single 450 nm photon is \( E = hv \), which is \( E = \frac{hc}{λ} \).
Therefore, the energy for n 450 nm photons would be \( E = n \cdot \frac{hc}{λ} \).
$\frac{(6.626 x 10^-34 Js)(3.00 x 10^8 m/s)}{450 x 10^-9 m}$ = 4.417 x 10-19 J
The minimum energy the photon should provide is 4.0 x 10-17 J.
The number of the photons required to provide this energy is:
\[ 4.0 x 10^{-17} J = n (4.417 x 10^{-19}) \]
$$n = \frac{4.0 x 10^{-17} J}{4.417 x 10^{-19} J}$$
= 91 photons
Note: Remember to convert the wavelength from nm to m!
S12.7
Boron and aluminum are the two elements that most commonly have less than 8 valence electrons so that they frequently fail to satisfy octet rule. Due to this reason, both boron and aluminum are reactive so they readily react with other compounds to complete an octet.
AlF3 will react with a fluoride anion to form the AlF4- anion, in which aluminum follows the octet rule.
Since the molecule does not satisfy the octet rule, it has resonance structures.
S13.11
We can use Born-Haber cycle to analyze reaction energy. The Born-Haber cycle deals with the formation of an ionic bond from the reaction of a group1&2 metal and a halogen. By using this cycle, we can calculate the energy that cannot be obtained directly.
\[ V_{0} = \frac{- (qNa+ + qcl-) (1- \frac{1}{n})} / {4πϵ0r_{0}} \]
$$-\frac{(1.602 x 10^{-19} C)^ 2} (6.022 x 10^{23} molecules/mol)(1-\frac{1}{10})}{(4 x 3.1416)(8.854 x 10^{-12} C^{2} /Nm^{2})(193 x 10^{-12} m)}$$
= -6.48 x 105 J/mol
= - 648 kJ/mol
- NaF
→ re = 1.93Å
→ n=10
- V0 denotes the potential energy associated with the most stable separation (re).
- re denotes the equilibrium bond length of the ion pair.
S14.14
The degeneracy of a rotational energy level is given by 2J+1, so that for J=10 the degeneracy is 2(10)+1 = 21
S2.70
\(c_{rms}\) is defined as
$$\sqrt{\frac{3RT}{M}}$$
- The crms is dependent on temperature at constant volume since T appears in the equation.
- The density does not affect the crms since density does not appear in the equation and M (molar mass) is not related to density.
- The crms is independent of the pressure at constant temperature. Even though the pressure changes, temperature stays constant and there is no change in crms.
- As the volume changes at constant temperature, as like the pressure above, there is no affect on crms since temperature stays the same.
- The size of the molecules does not affect the crms since it is not necessarily true that bigger molecules have higher molar mass. Different elements weigh different amounts.
S9.9
The approach for problems such as these is to determine the reaction order. This is useful because each reaction order has a very distinct linear relationship from which we can easily extrapolate other data. The absorbance of of a reactant is proportional to its concentration. Therefore, we can use the data to find reaction orders.
The easiest way to find the reaction order would be to plot the data using the three following rate laws:
- 0th order reaction: \( [A] = [A]_{0} - kt \rightarrow \) therefore, [A] is linearly related to t (Plot [A] vs t)
- 1st order reaction: \( \ln{[A]} = \ln{[A]_{0}} - kt \rightarrow \) therefore, ln[A] is linearly related to t (Plot ln[A] vs t)
- 2nd order reaction: \( \frac{1}{[A]} = \frac{1}{[A]_{0}} + kt \rightarrow \) therefore, \( \frac{1}{[A]} \) is linearly related to to (Plot 1/[A] vs t)
Plotting the three scenarios, we find that the data fits the first order reaction integrated rate law.
We can use a graphing utility to find the equation of the line. The slope of this line will be -k.
So, k = 0.0017 s-1

