Extra Credit 30
- Page ID
- 49190
Q2.56
Calculate the value of the crms of CH4 at 300K?
Relevant information:
- Molar mass of CH4 : M = 16.04 g mol-1
- Gas Constant : R = 8.3145 J mol-1 K-1
Q2.102
Calculate the ratio of the change in kinetic energy of a He gas sample which is heated from 200 K to 300K.
Q9.24
Q10.16
Derive a Michaelis-Menten equation for a competitive inhibition. (Hint: both the substrate S and the inhibitor I compete for the same active site of an enzyme E.)
Q11.14
Calculate the wavelengths in nm for a hydrogen-like atom, Li2+, for a transition from ni=2 to nf= 3 and 4. Then calculate the wavelengths for a hydrogen atom for the same transitions using the Rydberg constant and compare both answers.
Relevant information:
- Rydberg constant: 109,737 cm-1
Q12.5
Sometimes the concept of resonance is explained using an analogy such as a mule, which is a cross between a horse and a donkey. Compare this analogy to that of a griffins, which is a cross between a lion and an eagle. Explain which of the analogies is more appropriate in explaining the concept of resonance.
Q13.9
A Na+ ion is situated 4.0 angstroms away from the center of the CO2 molecule. Calculate the potential energy of interaction for an ion-induced dipole moment.
Relevant information:
- The polarizability of CO2 : \[2.91\alpha\times10^{-30}m^{3}\]
Q14.12
If each NMR scan for a dilute sample takes 5.0 minutes to generate a signal-to-noise (S/N) ratio of 1.5 then calculate how much time is required to generate a spectrum with a S/N ratio of 10.
Q2.80
About 30.5 mL of Helium gas effuses through a small hole in 4.00 minutes and a 10.0 mL mixture of NO and NO2 gasses effuses through the same hole under same conditions in same amount of time. Calculate the percent composition of NO and NO2 gas in the mixture.
Relevant information:
- Molar mass of Hydrogen gas = 4.003 g mol-1
- Molar mass of NO = 30.01 g mol-1
- Molar mass of NO2 = 46.0 g mol-1
Q9.22
The following data were collected for the reaction between hydrogen and nitric oxide at 500K:
\[2H_{2(g)}+2NO_{(g)}\rightarrow2H_{2}O_{(g)}+N_{2(g)}\]
| Experiment | [H2] (M) | [NO] (M) | Initial rate (M s-1) |
| 1 | 0.020 | 0.050 | \[4.2\times10^{-6}\] |
| 2 | 0.010 | 0.050 | \[2.1\times10^{-6}\] |
| 3 | 0.020 | 0.025 | \[1.05\times10^{-6}\] |
(a) What is the rate law for the reaction?
(b) Calculate the rate constant for the reaction.
(c) What happens to the rate law at very high and very low hydrogen concentrations?
\[rate=\frac{k_{1}[NO]^{2}[H_{2}]}{1+k_{2}[H_{2}]}\]
Answer Key
Q2.56: 683.0 m s-1
Q2.102: ratio is 1.5
Q9.24:
Q10.16: \[v_{o} = \frac{V_{max}[S]}{K_{s}(1+\frac{[I]}{K_{I}})+[S]}\]
Q11.14: For Li+2 ion wavelength were 72.9nm and 54.0nm. For H atom the wavelength were 656.11nm and 486.01nm.
Q12.5: Neither analogy encapsulates what resonance means.
Q13.9: V= -7.896 kJ mol-1
Q14.12: 220 mins or 3.667 hrs
Q2.80: NO is 55% and NO2 is 45%
Q9.22:(a) rate = k [NO]2 [H2] (b) 0.084M-2s-1 (c) For high hydrogen concentration rate = (k1 / k2) [NO]2 ; and for very low hydrogen concentration rate = k1 [NO]2 [H2]
Solutions
S2.56
We can calculate the crms of CH4 using equation \(c_{rms} = \sqrt{\dfrac{3RT}{M}}\) as we already know the molar mass and temperature is given to be 300K. The gas constant R used is 8.314 J K-1 mol-1.
\(c_{rms} = \sqrt{\dfrac{3RT}{M}} = \sqrt{\dfrac{3(8.314JK^{-1}mol^{-1})(300K)}{16.04\times10^{-3}kg mol^{-1}}} = 683.0\sqrt\frac{J}{kg}\times\sqrt\frac{kg m^{2}s^{2}}{1J} = 683.0\frac{m}{s}\)
S2.102
For one molecule the average translational kinetic energy is \[\bar{E}_{trans}=\frac{3}{2}k_{B}T\]. So now we just have to calculate the kinetic energy at different temperatures and take the ratio.
\[\frac{\bar{E}_{300}}{\bar{E}_{200}}=\frac{\frac{3}{2}k_{B}(300K)}{\frac{3}{2}k_{B}(200K)}=1.5\]
Therefore, % increase in kinetic energy is 50%
S9.24
S10.16
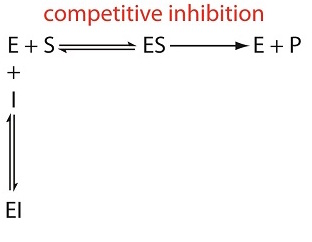
For competitive inhibition the substrate S and the Inhibitior I both compete for binding at the enzyme active site. Both the substrate and inhibitor cannot bind at the same time and only the enzyme-substrate complex [ES] can form a product.
\[v_{o} = [ES]k_{p}\]
\[V_{max} = [E]_{t}\times k_{p}\]
\[K_{s}= \frac{[E][S]}{[ES]} ; K_{I}= \frac{[E][I]}{[EI]}\]
\[\frac{v_{o}}{E_{t}}= \frac{[ES]k_{p}}{[E][ES][EI]}\]
\[\frac{v_{o}}{E_{t}\times k_{p}}= \frac{[ES]}{[E][ES][EI]}= \frac{v_{o}}{V_{max}}\]
now substituting for [ES] and [EI], we get
\[\frac{v_{o}}{V_{max}} = \frac{\frac{[E][S]}{K_{s}}}{[E]+\frac{[E][S]}{K_{s}}+ \frac{[E][I]}{K_{I}}}\]
Now we can simplify the equation by dividing both the denominator and numerator by [E]. So we get,
\[\frac{v_{o}}{V_{max}} = \frac{\frac{[S]}{K_{s}}}{1+\frac{[S]}{K_{s}}+ \frac{[I]}{K_{I}}}\]
For further simplification we can multiply both the denominator and numerator by Ks and we get,
\[\frac{v_{o}}{V_{max}} = \frac{[S]}{K_{s}+[S]+ \frac{K_{s}[I]}{K_{I}}}\]
We can convert this equation into Michales-Menten's form by rearranging the above equation:
\[v_{o} = \frac{V_{max}[S]}{K_{s}(1+\frac{[I]}{K_{I}})+[S]}\]
S11.14
We can use the Rydberg equation to calculate the wavelength of hydrogen like molecules and hydrogen. This equation takes in account the Z value which is 3 for Lithium and 1 for hydrogen atom.
\[\frac{1}{\lambda}= R_{H} Z^{2}(\frac{1}{n_{1}^{2}}-\frac{1}{n^{2}_{2}})\]
So for Li2+ we could solve as follow:
when ni = 2 and nf = 3
\[\frac{1}{\lambda}= 109,737cm^{-1} (3^{2})(\frac{1}{2^{2}}-\frac{1}{3^{2}})\]
\[\frac{1}{\lambda}= 137171.25cm^{-1}\]
\[\lambda=7.290\times10^{-6}cm=72.90 nm\]
when ni = 2 and nf = 4
\[\frac{1}{\lambda}= 109,737cm^{-1} (3^{2})(\frac{1}{2^{2}}-\frac{1}{4^{2}})\]
\[\frac{1}{\lambda}= 185181cm^{-1}\]
\[\lambda=5.4001\times10^{-6}cm=54.00 nm\]
For Hydrogen atom, Z=1.
when ni = 2 and nf = 3
\[\frac{1}{\lambda}= 109,737cm^{-1} (1^{2})(\frac{1}{2^{2}}-\frac{1}{3^{2}})\]
\[\frac{1}{\lambda}= 15241.25cm^{-1}\]
\[\lambda=6.5611\times10^{-5}cm=656.11nm\]
when ni = 2 and nf = 4
\[\frac{1}{\lambda}= 109,737cm^{-1} (1^{2})(\frac{1}{2^{2}}-\frac{1}{4^{2}})\]
\[\frac{1}{\lambda}= 20575.6875cm^{-1}\]
\[\lambda=4.8601\times10^{-5}cm=486.01nm\]
As we see from above calculations there is a huge difference in the wavelength for hydrogen and lithium ion. All of the above transitions for H are in the visible region while Li2+ is in ultraviolet region. There is such huge due to the Z2 factor in the equation.
S12.5
Resonance is a way of describing the delocalized pi system in a molecule, where the molecule can have more than 1 possible Lewis dot structure. Each of the resonance structures are equally possible and the molecule with such a delocalized system usually keeps altering between its resonance structure. We can also draw a hybrid structure of those resonance forms of molecule which is intermediate form of the molecule while transitioning from one resonance form to other. Therefore the analogy of a mule which is a hybrid of a horse and a donkey is fine to use when explaining the hybrid structure of the resonance. It is not exactly correct to use this analogy as the mule cannot turn back into a donkey or horse. Similarly we cannot really use the analogy of a griffin either which is a hybrid of a lion and an eagle.
S13.9
So we know from the question that
\[r=4.0 angstroms =4.0\times10^{-10}m\] and \[\alpha(CO_{2})= 2.91\times10^{-30}m^{3}\]
We can use the equation \[V= -\frac{1}{2}\frac{\alpha q^{2}}{4\pi\epsilon_{o}r^{4}}\] to calculate the induced dipole moment
\[V= -\frac{1}{2}\frac{(2.91\times10^{-30}m^{3}) (1.602\times10^{-19}C)^{2}}{4\pi(8.854\times10^{-12}C^{2}N^{-1}m^{-2})(4.0\times10^{-10}m)^{4}} \frac{J}{N\times m}\]
\[V=-1.31\times10^{-20}J\]
If we multiply the value by Avagadro's number we get:
\[V=(-1.31\times10^{-20}J)(6.023\times10^{23}mol^{-1}) = -7.896\times10^{3}Jmol^{-1}\]
\[V=-7.896 kJ mol^{-1}\]
S14.12
After n scans the single intensity will increase by a factor of n, while the noise will increase by a factor of n1/2 , so that using
\[\frac{(S)}{(N)_{n}}=\frac{nS}{\sqrt{n}N}=\sqrt{n}\frac{(S)}{(N)_{1}}\]
\[\sqrt{n}=\frac{(S/N)_{n}}{(S/N)_{1}}=\frac{10}{1.5}=6.667\]
\[n=44\]
So getting 44 scans at 5.0 minutes per scan will require:
\[44\times5min=220mins=3.667hrs\]
S2.80
For this question we use Graham's Law of Effusion which states that the rate of effusion of a gaseous substance is inversely proportional to the square root of its molar mass. So,
\[\frac{r_{1}}{r_{2}}=\sqrt\frac{M_{2}}{M_{1}}\]
Now we just have to plug in all the values and we will get the following equation:
\[(\frac{\frac{30.5mL}{4.00min}}{\frac{10.0mL}{4.00min}})=\sqrt\frac{M_{mix}}{1.00g\times mol^{-1}}\]
We square both the sides and multiply the ratio of rates with the molar mass of Hydrogen gas to obtain Mmix .
\[M_{mix}=(\frac{\frac{30.5mL}{4.00min}}{\frac{10.0mL}{4.00min}})^{2}\times(4.003g\times mol^{-1})=37.238\frac{g}{mol}\]
Let xNO be the mole fraction for NO and xNO2 be the mole fraction of NO2 . So that
\[x_{NO}+x_{NO_{2}}=1\]
\[x_{NO_{2}}=(1-x_{NO})\]
The effective molar mass of the mixture can be obtained from the mole fractions and molar masses of its components:
\[x_{NO}M_{NO}+x_{NO_{2}}M_{NO_{2}}=M_{mix}\]
Subsituting \[x_{NO_{2}}=(1-x_{NO})\] into the above equation gives us:
\[x_{NO}M_{NO}+(1-x_{NO})M_{NO_{2}}=M_{mix}\]
Now plug in the given values and the molar mass of the mixture obtained from above
\[x_{NO}(30.01\frac{g}{mol}) +(1-x_{NO})(46.0\frac{g}{mol})=37.238\frac{g}{mol}\]
\[30.01x_{NO}+46.0- 46.0x_{NO}=37.238\]
\[15.99x_{NO}=8.762\]
\[x_{NO}=0.55\]
Therefore the mole fraction \[x_{NO_{2}}=0.45\]
So the % of NO = 55% and % of CO2 = 45%
S9.22
(a) For experiment 1 and 2, the concentration of H2 is decreased by one-half and the concentration for NO is constant, the rate of reaction decreases by half also.
\[\frac{rate2}{rate1}=\frac{2.1\times10^{-6}}{4.2\times10^{-6}}=\frac{1}{2}\]
Therefore, we can say that the reaction order for H2 is first order as the change in its concentration is directly proportional to the change in the initial rate of the reaction. Thus, \[rate\sim k[H_{2}]\]
For experiment 1 and 3, the concentration of H2 is kept constant and the concentration of NO has decreased by one-half. The initial rate of the reaction decreases by one-forth.
\[\frac{rate3}{rate1}=\frac{1.05\times10^{-6}}{4.2\times10^{-6}}=\frac{1}{4}\]
Therefore, we can say that the reaction order for NO is second order because the initial rate is proportional to the squared concentration of NO. Thus, \[rate\sim k[NO]^{2}\]
Therefore, the rate law will be \[rate=k[NO]^{2}[H_{2}]\]
(b) To calculate the rate constant k, we can use data from any experiments (1,2 or3) and plug in the values in the above derived rate law.
Using Experiment 1 to calculate k,
\[4.2\times10^{-6}Ms^{-1}=k[0.050M]^{2}[0.020M]\]
\[k=\frac{4.2\times10^{-6}Ms^{-1}}{[0.050M]^{2}[0.020M]}=0.084M^{-2}s^{-1}\]
(c) At very high concentration of H2, \[k_{2} [H_{2}]\gg1\] Therefore, the rate law becomes
\[rate=\frac{k_{1}[NO]^{2}[H_{2}]}{k_{2}[H_{2}]}=\frac{k_{1}}{k_{2}}[NO]^{2}\]
At very low hydrogen concentrations, \[k_{2}[H_{2}]\ll1\] Therefore, the rate will be
\[rate=k_{1}[NO]^{2}[H_{2}]\]

