Extra Credit 7
- Page ID
- 82966
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.5B
- H2O2+Sn2+⟶H2O+Sn4+
- PbO2+Hg⟶Hg22++Pb2+
- Al+Cr2O72-⟶Al3++Cr3+
Q17.1.6
Identify the species that undergoes oxidation, the species that undergoes reduction, the oxidizing agent, and the reducing agent in each of the reactions of the previous problem.
S17.1.6
An oxidizing agent is the substance that causes the oxidation in another substance. Common oxidizing agents include oxygen, hydrogen peroxide and halogens. A reducing agent is a substance that causes another substance to reduce. So to identify an oxidizing agent, simply look at the oxidation number of an atom before and after the reaction. If the oxidation number is greater in the product, then it lost electrons and the substance was oxidized. If the oxidation number is less, then it gained electrons and was reduced. The substance that is reduced in a reaction is the oxidizing agent because it gains electrons. The substance that is oxidized in a reaction is the reducing agent because it lost electrons.
| Name | Oxidation Number | Change in Electrons |
|---|---|---|
| Oxidation | increases | electrons are lost |
| Reduction | decreases | electrons are gained |
| Oxidizing agent | decreases | electrons are gained |
| Reducing agent | increases | electrons are lost |
1.
- Sn2+⟶2e−+Sn4+ (oxidation)
- H2O2+2H++2e−⟶2H2O (reduction)
In the first reaction, the oxidation state of Sn goes from 2+ to 4+, it is therefore oxidized as it is losing two electrons. H2O2 is reduced as it is countering the loss of electrons from Sn. H2O2 will become H2O and will be balanced with an additional water molecule and 2e- and 2H+ ions. In this equation, H2O2 is the oxidizing agent because it is gaining electrons and it is bringing about the oxidation of Sn. Sn, in turn, is the reducing agent as it is losing electrons and causing H2O2 to gain electrons.
2.
- PbO2+2e−+4H+⟶2H2O+Pb2+ (reduction)
- 2Hg⟶Hg2+2+2e−(oxidation)
In the second reaction, the oxidation state of Hg goes from 2Hg to Hg22+, meaning it will lose two electrons. PbO2 is reduced, it counters the loss of electrons from Hg. The oxidizing agent was PbO2 as it is gaining electrons and is bringing about the oxidation of Hg. Comparatively, Hg is the reducing agent because it is losing electrons and causing PbO2 to be reduced.
3.
- [ Al⟶Al3++3e−] x2 to cancel electrons from both half-reactions (oxidation)
- 14H++6e−+Cr2O2−7⟶2Cr3++7H2O (reduction)
In the third reaction, the oxidation state of Al goes from 0 to +3, meaning it loses three electrons and is oxidized. Cr goes from Cr2O72- to Cr3+, it was balanced with water but in the overall reaction, Cr2O72- gained electrons (reduced). Cr2O72- is the oxidizing agent because it oxidizes Al or causes it to lose its electrons. Al has the opposite effect as it is the reducing agent and causes Cr2O72- to gain electrons.
*phase II - correct
Q19.1.5
Which of the following elements is most likely to be used to prepare La by the reduction of La2O: Al, C, or Fe? Why?
S19.1.5
The strength of reducers and oxidizers depends upon the thermodynamic favor ability of their reactions. The strongest elemental reducing agent is lithium, which is not the least electronegative element. When Li acts as a reducing agent, metallic bonds are broken and one electron is removed from each La atom. These processes are endothermic.
Populated orbitals with high energy mean the substance tends to be a reducing agent, while unpopulated orbitals with low energy mean the substance tends to be oxidizing.
The Relative Strengths of Common Oxidizing Agents and Reducing Agents
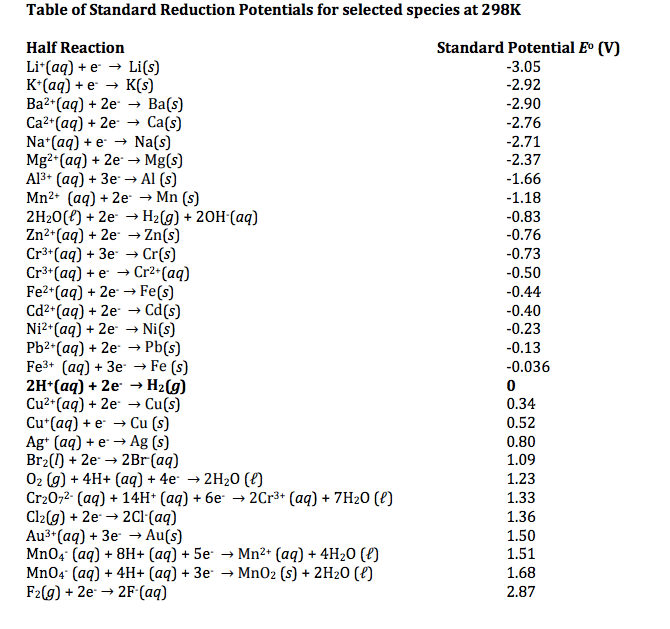
Based on the reduction half-reaction chart, Al is the strongest reducing agent with a E°(V) value of -1.66, Fe has a E°(V) value of 2.87 making it a good oxidizing agent, and C has a E°(V) value of +0.207. Between the three options, Al, C, and Fe, we see that the strongest reducing agent is Al. Al would most likely be used to prepare La by the reduction of La2O3 because Al is the strongest reducing agent. Al is used because it is the strongest reducing agent and the only option listed that can provide sufficient driving force to convert La(III) into La.
*phase II - correct
Q19.2.5
- [Co(en)2(NO2)Cl]+
- [Co(en)2Cl2]+
- [Pt(NH3)2Cl4]
- [Cr(en)3]3+
- [Pt(NH3)2Cl2]
Q19.2.7
Name each of the compounds or ions given in Exercise Q19.2.5.
S19.2.7
1. [Co(en)2(NO2)Cl]+ chlorobis(ethylenediamine)nitrocobalt(III) ion
Chlorobis comes from Cl, there is only one Cl which means there will be no prefix added. Ethylenediamine is the long form of (en), nitrocobalt is the combination of nitrogen and cobalt (NO2) and (Co). It is the third isotope of Cobalt.
2. [Co(en)2Cl2]+ dichlorobis(ethylenediamine)cobalt(III) ion
Dichlorobis comes from Cl2, the di is used to diffrentiate that there are two molecules. Enylenediamine is the short form of (en). Once again, the third isotope of Cobalt is used, but it is alone unlike the previous compound.
3. Pt(NH3)2Cl4 diamminetetrachloroplatinum(IV)
The ammine molecule is NH3, there are two molecules hence the prefix di. There are four Cl molecules, giving Chlorine the prefix tetra. It is combined with platinum (IV), the fourth isotope of Platinum.
4. [Cr(en)3]3+ tris(ethylenediamine)chromium(III) ion
Tris is used because there are three enylenediamine molecules. Chromium (III) is simply naming the molecule, Cr.
5. Pt(NH3)2Cl2 diamminedichloroplatinum(II)
Once again, ammine is NH3 and because there are two molecules, it is diammine. Dichloro is because there are two Cl molecules. Lastly, the third isotope of platinum is used to balance the compound.
*phase II - correct
Q12.3.20
The rate constant for the first-order decomposition at 45 °C of dinitrogen pentoxide, N2O5, dissolved in chloroform, CHCl3, is 6.2 × 10-4 min-1.
2N2O5⟶4NO2+O2
What is the rate of the reaction when [N2O5] = 0.40 M?
S12.3.20
Given:
Rate constant (k): 6.2 × 10−4 min−1
Order of N2O5: first order
Concentration of N2O5: 0.40 M
Wanted:
Rate of the reaction
rate=k[A]m[B]n shows that the rate of the reactants depend on the concentration of the reactants. The values of m and n are reaction order with respect to A and B respectively. Order does not depend on stoichiometric values, but rather trials. The rate constant (k) does not depend on concentrations of the reactants (A and B), but is affected by surface area and temperature.
rate = k [reactants]1 Since the overall reaction is first-order N2O5 must be to the first power. k is the rate constant given in the problem (6.2 x 10-4 min-1)
rate = (6.2 x 10-4 min-1) (0.4 M)1
rate = 0.000248 M/min
or 2.5 M/min
*phase II - should be 2.5x10-4 M/min
Q12.6.11
The reaction of CO with Cl2 gives phosgene (COCl2), a nerve gas that was used in World War I. Use the mechanism shown here to complete the following exercises:
- Cl2(g)⇌2Cl(g) (fast, k1 represents the forward rate constant, k-1 the reverse rate constant)
- CO(g)+Cl(g)⟶COCl(g) (slow, k2 the rate constant)
- COCl(g)+Cl(g)⟶COCl2(g) (fast, k3 the rate constant)
- Write the overall reaction.
- Identify all intermediates.
- Write the rate law for each elementary reaction.
- Write the overall rate law expression.
S12.6.11
![]()
1. The overall reaction of CO and Cl2
CO(g)+Cl2(g)⟶COCl2(g)
2. Intermediates:
Reaction intermediates are formed in one step by later consumed in a future step. They are not present in the overall reaction. Looking at the three elementary steps given above, we can see that COCl and Cl will be the intermediates as they are not present in the overall reaction.
3. Rate law for each elementary reaction:
Determine the rate law for each reaction by taking in consideration the k (rate constant) for each reaction and include the reactants.
rate law for step 1:
rate = k1 [Cl2] = k-1 [Cl]2
*phase II - should be k-1[Cl]2
rate law for step 2:
rate = k2 [CO][Cl]
rate law for step 3:
rate = k3 [COCl][Cl]
4. Overall rate law expression
We put the elementary rate laws determined for each reaction together to make the overall rate law expression.
k1 [Cl2] = k-1 [Cl]2
= k1 [Cl2] = k-1 [Cl]2
= k1 [Cl2]/k-1 = [Cl]2
= ( k1 [Cl2]/k-1 )1/2 = [Cl]
Substitute ( k1 [Cl2]/k-1 )1/2 = [Cl] into second step
k [CO][Cl]
= k2 ( k1 [Cl2]/k-1 )1/2 [CO]
= k [Cl2]1/2 [CO]
k3 [COCl][Cl]
= k3 [COCl] ( k1 [Cl2]/k-1 )1/2
= (k3(k1/k-1)1/2) [COCl][Cl2]1/2
= (k3(k1/k-1)1/2) [COCl][Cl] (COCl and Cl are intermediates)
Overall rate law expression: = (k3(k1/k-1)1/2)
*phase II - incorrect
First use the equation
rate = k2 [CO][Cl]
because it is the slowest step. Because Cl is an intermediate, it can't be in the rate law. Substitute using the rate law for the first elementary reaction to get
[Cl]= sqrt (k1[Cl2] / k-1)
substitute into the first equation to get final answer.
rate = k2 [CO] sqrt(k1[Cl2] / k-1)
Q21.4.23
Plutonium was detected in trace amounts in natural uranium deposits by Glenn Seaborg and his associates in 1941. They proposed that the source of this 239Pu was the capture of neutrons by 238U nuclei. Why is this plutonium not likely to have been trapped at the time the solar system formed 4.7 × 109 years ago?
S21.4.23
239Pu, an isotope of Plutonium, has a half-life of 24,110 years. Fission of an atom of uranium-235 will produce 2-3 neutrons which can be absorbed by uranium-238 to produce plutonium-239. This shows that Pu-239 could not remain since the formation of the earth. Because of this, present plutonium could not have been formed with the 238Uranium. Plutonium now present would not be able to form with the uranium originally available. You can also calculate the number of half lives that it has undergone.
*phase II - correct
Q20.3.11
Sulfate is reduced to HS− in the presence of glucose, which is oxidized to bicarbonate. Write the two half-reactions corresponding to this process. What is the equation for the overall reaction?
S20.3.11
Sulfate (SO42-) is first reduced to HS- in the presence of glucose (C6H12O6).
reduction: SO24-(aq)+9H+(aq)+8e-→HS-(aq)+4H2O(l)
Second, glucose is oxidized to bicarbonate.
oxidation:C6H12O6(aq)+12H2O(l)→6HCO3-(g)+30H+(aq)+24e-
After multiplying the reduction reaction by 3 to cancel the electrons from both half-reactions, subtracting the H+ from both sides, and canceling out the H2O we get the following half-reactions:
3SO42-(aq) → 3HS-(aq) + 12H2O(l)
C6H12O6(aq) + 12H2O(l) → 6HCO3-(g) + 3H(aq)
Finally, when combining the two half-reactions we get the following overall reaction:
overall: C6H12O6(aq)+3SO42-(aq)→6HCO3-(g)+3H+(aq)+3HS-(aq)
*phase II - correct
Q20.5.22 
Mn(III) can disproportionate (both oxidize and reduce itself) by means of the following half-reactions:
Mn3+(aq) + e− → Mn2+(aq) E°=1.51 V
Mn3+(aq) + 2H2O(l) → MnO2(s) + 4H+(aq) + e− E°=0.95 V
- What is E° for the disproportionation reaction?
- Is disproportionation more or less thermodynamically favored at low pH than at pH 7.0? Explain your answer.
- How could you prevent the disproportionation reaction from occurring?
S20.5.22
1. What is E° for the disproportionation reaction?
E°cell = E°cathode - E°anode
The cathode is where reduction occurs and the anode is where oxidation occurs. The reduction reaction is Mn3+(aq)+e−→Mn2+(aq) (E°=1.51 V) and the oxidation reaction is Mn3+(aq)+2H2O(l)→MnO2(s)+4H+(aq)+e− (E°=0.95 V).
E°cell = 1.51 V - 0.95 V
E°cell = 0.56V
It is spontaneous.
2. Is disproportionation more or less thermodynamically favored at low pH than at pH 7.0?
Disproportionation is more thermodynamically favored at a low pH rather than at pH 7.0. This is due to the fact that as the pH increases, the rate for the forward reaction will slow because the radicals will become more persistent.
3. How could you prevent the disproportionation reaction from occurring?
Disproportionation reactions are those in which a substance is simultaneously oxidized and reduced, giving two different products. They occur because one state is unstable when it comes to both its reduced and oxidized form which is what makes it disproportionate. The disproportionate reaction occurs to gain more stability. Water is needed for disproportionation reactions to occur, it is simple to prevent them by removing the water. The key to disproportionation reactions are the oxidation states of oxygen because this will be needed to reduce and oxidize Mn (III), the oxygen therefore is key.
*phase II - correct

