Extra Credit 6
- Page ID
- 82965
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.5B
Balance the following in acidic solution:
- H2O2 + Sn2+ ⟶ H2O + Sn4+
- PbO2+Hg ⟶ Hg22++ Pb2
- Al + Cr2O72-⟶ Al3+ + Cr3+
Solution: Q17.1.5B
1. Given: H2O2 + Sn2+ ⟶ H2O + Sn4+
Asked For: To balance the equation in an acidic solution
Strategy:
Step 1: Split the equation into half-reactions
One half reaction can be the oxidation reaction and the other is the reduction reaction.
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Sn2+ ⟶ Sn4+ | H2O2 ⟶ H2O |
Step 2: Balance oxygen with H2O(l)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Sn2+ ⟶ Sn4+ | H2O2 ⟶ H2O + H2O |
Step 3: Balance hydrogen with H+(aq)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Sn2+ ⟶ Sn4+ | H2O2 + 2H+ ⟶ H2O + H2O |
Step 4: Balance charge with e-
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Sn2+ ⟶ Sn4+ + 2e- | H2O2 + 2H+ + 2e- ⟶ H2O + H2O |
Step 5: Combine the half reactions
Sn2+ + H2O2 + 2H+ + 2e- ⟶ H2O + H2O + Sn4+ + 2e-
Step 6: Reduce the balanced equation
Sn2+ + H2O2 + 2H+ + 2e- ⟶ H2O + H2O + Sn4+ + 2e- Cancel 2e-
Sn2+ + H2O2 + 2H+ ⟶ 2H2O + Sn4+ Combine H2O (l) to form 2H2O (l)
Final Answer:
Sn2+ + H2O2 + 2H+ ⟶ 2H2O + Sn4+
2. Given: PbO2 + Hg ⟶ Hg22++ Pb2
Asked For: To balance the equation in an acidic solution
Strategy:
Step 1: Split the equation into half-reactions
One half reaction can be the oxidation reaction and the other is the reduction reaction.
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Hg ⟶ Hg22+ | PbO2 ⟶ Pb2+ |
Step 2: Balance all elements other than O and H
| Oxidation Half Reaction: | Reduction Half Reaction: |
| 2Hg ⟶ Hg22+ | PbO2 ⟶ Pb2+ |
Step 3: Balance oxygen with H2O(l)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| 2Hg ⟶ Hg22+ | PbO2 ⟶ Pb2+ + 2H2O |
Step 4: Balance hydrogen with H+(aq)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| 2Hg ⟶ Hg22+ | PbO2 + 4H+⟶ Pb2+ + 2H2O |
Step 5: Balance charge with e-
| Oxidation Half Reaction: | Reduction Half Reaction: |
| 2Hg ⟶ Hg22+ + 2e- | 2e- + PbO2 + 4H+⟶ Pb2+ + 2H2O |
Step 6: Combine the half reactions
2Hg + 2e- + PbO2 + 4H+⟶ Pb2+ + 2H2O + Hg22+ + 2e-
Step 7: Reduce the balanced equation
2Hg + 2e- + PbO2 + 4H+⟶ Pb2+ + 2H2O + Hg22+ + 2e- Cancel out the 2e-
Final Answer:
2Hg + PbO2 + 4H+⟶ Pb2+ + 2H2O + Hg22+
3. Given: Al + Cr2O72-⟶ Al3+ + Cr3+
Asked For: To balance the equation in an acidic solution
Strategy:
Step 1: Split the equation into half-reactions
One half reaction can be the oxidation reaction and the other is the reduction reaction.
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Al ⟶ Al3+ | Cr2O72- ⟶ Cr3+ |
Step 2: Balance all elements other than O and H
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Al ⟶ Al3+ | Cr2O72- ⟶ 2Cr3+ |
Step 3: Balance oxygen with H2O(l)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Al ⟶ Al3+ | Cr2O72- ⟶ 2Cr3+ + 7H2O |
Step 4: Balance hydrogen with H+(aq)
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Al ⟶ Al3+ | 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O |
Step 5: Balance charge with e-
| Oxidation Half Reaction: | Reduction Half Reaction: |
| Al ⟶ Al3+ + 3e- | 6e- + 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O |
Step 6: Balance the electrons
| Oxidation Half Reaction: | Reduction Half Reaction: |
| 2(Al ⟶ Al3+ + 3e-) | 6e- + 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O |
| 2Al ⟶ 2Al3+ + 6e- | 6e- + 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O |
Step 7: Combine the half reactions
2Al + 6e- + 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O + 2Al3+ + 6e-
Step 8: Reduce the balanced equation
2Al + 6e- + 14H+ + Cr2O72- ⟶ 2Cr3+ + 7H2O + 2Al3+ + 6e- Cancel the 6e-
Final Answer:
14H+ + 2Al + Cr2O72- ⟶ 2Cr3+ + 2Al3++ 7H2O
Q19.1.4
Why are the lanthanoid elements not found in nature in their elemental forms?
Solution: Q19.1.4
Lanthanides are the chemical elements found in Row 6 of the periodic table between Groups 3 and 4. They follow lanthanum (La), element #57, which accounts for their family name. The lanthanides include the metals cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium(Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). One thing about lanthanides is that they are not so rare, but they are very alike. Most of the lanthanides occur together in nature, and they are very difficult to separate from each other. They are not found in their elemental form in nature because of where they are found and their characterisitics. They are found in the Earth's crust which makes it incredibly difficult to extract.
Q19.2.6
Name each of the compounds or ions given in Exercise Q19.2.3, including the oxidation state of the metal.
- [Co(CO3)3]3-
- [Cu(NH3)4]2+
- [Co(NH3)4Br2]2(SO4)
- [Pt(NH3)4][PtCl4]
- [Cr(en)3](NO3)3
- [Pd(NH3)2Br2]
- K3[Cu(Cl)5]
- [Zn(NH3)2Cl2]


Solution: 19.2.6
All compound names must be written in alphabetical order!
1. Given: [Co(CO3)3]3-
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Co(CO3)3]3- → (CO3)3 = 3- carbonato
Step 2: Write out prefixes and name of metal; add -ate at the end of the transition metal because the complex is an anion.
Tricarbonatocobaltate
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
[Co(CO3)3]3- Co=x 3CO-3=3(−2)
3(−2)+x=−3 x=+3 So the oxidation number of Co is +3
Step 4: Combine the name and oxidation number into one name
Tricarbonatocobaltate (III) ion
2. Given: [Cu(NH3)4]2+
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Cu(NH3)4]2+ → (NH3)4 = 4-ammine
Step 2: Write out prefixes and name of metal
tetraamminecopper
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
[Cu(NH3)4]2+ Cu=x 4NH3=4(0)
4(0)+x=2 x=+2 So the oxidation number of Cu is +2
Step 4: Combine the name and oxidation number into one name
tetraamminecopper(II) ion
3. Given: [Co(NH3)4Br2]2(SO4)
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Co(NH3)4Br2]2(SO4) → (NH3)4 = 4 - ammine , Br2 = 2-bromo
Step 2: Write out prefixes and name of metal
Tetraamminedibromocobalt
Step 3: Determine the oxidation number of the ligand and anion
[Co(NH3)4Br2]2(SO4) Co=x 8NH3=4(0) Br2=2(−1) SO4=−2
2[4(0)+2(−1)+x]+−2=0 −6+2x=0 x=+3 So the oxidation number of Co is +3
Step 4: Combine the name and oxidation number into one name
tetraamminedibromocobalt(III) sulfate
4. Given: [Pt(NH3)4][PtCl4]
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands in the cation and anion
[Pt(NH3)4][PtCl4] → Cation: (NH3)4 = 4-ammine
Anion: Cl4 = 4-chloro
Step 2: Write out prefixes and name of metal; add -ate at the end of the transition metal because the complex is an anion.
Cation: tetraamineplatinum
Anion: tetrachloroplatinate
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
Cation: [Pt(NH3)4] Pt=x 4NH3=4(0)
4(0)+x=
Anion: [PtCl4] Pt=y 4Cl=4(−1)
4(−1)+y=
4(0)+ x + 4(-1)+ y= 0 x+y+−4=0 x+y=4
Pt has common oxidation states of +2 and +4. Using this, we see that Pt is equal in both the anode and cathode which must be equal to +2.
Step 4: Combine the name and oxidation number into one name
tetraammineplatinum(II) tetrachloroplatinate(II)
5. Given: [Cr(en)3](NO3)3
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Cr(en)3](NO3)3 → (en)3 = 3-en
Step 2: Write out prefixes and name of metal
tris-(en)chromium
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
[Cr(en)3](NO3)3 Cr=x 3(en)=3(0) 3NO3=3(0)
3(0)+x=−3 x=+3 So the oxidation number of Cr is +3
Step 4: Combine the name and oxidation number into one name
tris-(ethylenediamine)chromium(III) nitrate
6. Given: [Pd(NH3)2Br2]
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Pd(NH3)2Br2] → (NH3)2 = 2-ammine , Br2= 2-bromo
Step 2: Write out prefixes and name of metal
Diamminedibromopalladium
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
[Pd(NH3)2Br2] Pd=x 2NH3=2(0) 2Br=2(−1)
2(0)+2(−1)+x=0 x=+2 So the oxidation number of Pd is +2
Step 4: Combine the name and oxidation number into one name
diaminedibromopalladium(II)
7. Given: K3[Cu(Cl)5]
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
K3[Cu(Cl)5] → (Cl)5 = 5-chloro
Step 2: Write out prefixes and name of metal with the suffix; add -ate at the end of the transition metal because the complex is an anion.
Pentachlorocuprate
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
K3[Cu(Cl)5] 3K=+3 Cu=x 5Cl=5(−1)
5(−1)+x+3(1)=0 x=+2 So the oxidation number of Cu is +2
Step 4: Combine the name and oxidation number into one name
potassium pentachlorocuprate(II)
8. Given: [Zn(NH3)2Cl2]
Asked for: Name compound with oxidation state of metals
Strategy:
Step 1: Identify the ligands
[Zn(NH3)2Cl2] → (NH3)2 = 2-ammine , Cl2 = 2-chloro
Step 2: Write out prefixes and name of metal
Diamminedichlorozinc
Step 3: Determine the oxidation number of the ligand by counting charge and ion charge
[Zn(NH3)2Cl2] Zn=x 3NH=3(0) 2Cl=2(−1)
2(0)+2(−1)+x=0 x=+2 So the oxidation number is +2
Step 4: Combine the name and oxidation number into one name
Diamminedichlorozinc (II)
Q12.3.19
For the reaction Q⟶W+XQ⟶W+X, the following data were obtained at 30 °C:
| [Q]initial (M) | 0.170 | 0.212 | 0.357 |
|---|---|---|---|
| Rate (mol/L/s) | 6.68 × 10−3 | 1.04 × 10−2 | 2.94 × 10−2 |
- What is the order of the reaction with respect to [Q], and what is the rate equation?
- What is the rate constant?
Solution: Q12.3.19
1. What is the order of the reaction with respect to [Q], and what is the rate equation?
Step 1: Take ratio of the rate divided by concentration using this equation → rate/[Q]=k
6.68x10-3/.17=.0393 1.04×10-2/.212=.0491 2.94×10-2/.357=.0824
None of the ratios above are the same. This means that this is NOT a first order reaction.
Step 2: Use 2nd order equation → rate/[Q]2=k
6.68x10−3/.172=.231 1.04×10−2/.2122=.231 2.94×10−2/.3572=.231
These ratio values are all the same, so this is a second order reaction.
The rate equation is: rate=k[Q]2
For a second order reaction, the units for the rate constant (k) is 1/Ms
2. The ratio value that we got is the rate constant: .231 1/Ms
Q12.6.10
Experiments were conducted to study the rate of the reaction represented by this equation.
2NO(g) + 2H2(g) ⟶ N2(g) + 2H2O(g)
Initial concentrations and rates of reaction are given here.
| Experiment | Initial Concentration [NO] (mol/L) | Initial Concentration, [H2] (mol/L) | Initial Rate of Formation of N2(mol/L min) |
|---|---|---|---|
| 1 | 0.0060 | 0.0010 | 1.8 × 10−4 |
| 2 | 0.0060 | 0.0020 | 3.6 × 10−4 |
| 3 | 0.0010 | 0.0060 | 0.30 × 10−4 |
| 4 | 0.0020 | 0.0060 | 1.2 × 10−4 |
Consider the following questions:
- Determine the order for each of the reactants, NO and H2, from the data given and show your reasoning.
- Write the overall rate law for the reaction.
- Calculate the value of the rate constant, k, for the reaction. Include units.
- For experiment 2, calculate the concentration of NO remaining when exactly one-half of the original amount of H2 had been consumed.
- The following sequence of elementary steps is a proposed mechanism for the reaction.
Step 1: NO + NO ⇌ N2O2
Step 2: N2O2 + H2 ⇌ H2O + N2O
Step 3: N2O + H2 ⇌ N2 + H2O
Based on the data presented, which of these is the rate determining step? Show that the mechanism is consistent with the observed rate law for the reaction and the overall stoichiometry of the reaction.
Solution: Q12.6.10
Step 1: Determine the order of NO by taking the change in concentration and comparing it to the change in rate.
0.002/.001 = 2 0.3x10-4/1.2x10-4 = 4.
The rate of the reaction quadruples and the concentration doubles. This tells us that this is a second order reaction.
Step 2: Determine the order of H2 by taking the change in concentration and comparing it to the change in rate.
0.002/0.001 = 2 3.6x10-4/1.8x10-4 = 2.
The concentration of hydrogen doubles and so does the rate. This tells us this is a first order reaction.
Step 3: Write the rate law for the entire equation.
Rate=K[H2][NO]2
Step 4: Calculate the value of the rate constant, k, for the reaction. Include units.
In order to determine the rate constant, you must choose values from the chart given and plug them into the rate law equation.
1.8x10-4 mol/L·min = k(.001 mol/L)(.006mol/L)2
1.8x10-4 mol/L·min= k(3.6x10-8 mol3/L3)
(1.8x10-4 mol/L·min)/ (3.6x10-8 mol3 L3)= 5000 mol-2 L-2 min-1
k = 5.0 × 103 mol−2 L−2 min−1
Step 5: For experiment 2, calculate the concentration of NO remaining when exactly one-half of the original amount of H2 had been consumed.
[NO] and [H2] have a 1:1 molar ratio.
If 0.001 moles of hydrogen decay after one half-life, then 0.001 moles of NO decay after one hydrogen half-life.
If the sample of NO was 0.006, then the remaining amount would be 0.006-0.001=0.005 M NO.
Step 6:
Step 1: NO + NO ⇌ N2O2
Step 2: N2O2 + H2 ⇌ H2O + N2O
Step 3: N2O + H2 ⇌ N2 + H2O
Step II is the rate-determining step.
Step II: rate= k2[N2O2][H2]
N2O2 is an intermediate and does not appear in the overall reaction or overall rate law. Therefore, we must substitute N2O2 with the proper concentrations.
rate= k1[NO]2/ k-1[N2O2]
Rearrange the equation so it equal to [N2O2].
[N2O2]= k1[NO]2/k-1
Substitute [N2O2] for the new equation.
rate= k2(k1[NO]2/k-1)[H2]
The rate constants will combine to form regular k.
Overall rate law:
rate= k[NO]2[H2]
This reaction corresponds to the observed rate law.
Combine steps 1 and 2 with step 3 (in Step 6), which occurs by supposition in a rapid fashion, to give the appropriate stoichiometry.
Q21.4.22
A laboratory investigation shows that a sample of uranium ore contains 5.37 mg of 238U and 2.52 mg of 206Pb. Calculate the age of the ore. The half-life of 238U is 4.5 × 109 yr.
Solution: Q21.4.22
Step 1: Determine the percentage of U in the given sample.
→ 5.37/(5.37+2.52)= 0.6806
→ 0.6806 x 100% = 68.06% (This is the percentage of uranium in the sample)
Step 2: Use the percentage found in the half life formula.
-Use 1 as the initial (Ao) mass (100% U)
-Use 0.6806 as the current (A) mass (68.06% left)
-Use 4.5x109 years as the given half life (h)
-Solve for t
Use half life formula: A= A0(.5 t/(h^2))
.6808 =.5t/((4.5x10^9)^2)
Step 3: Solve for t
→ ln(.6808)=ln(.5t/((4.5x10^9)^2))
→ −.38449=(t/((4.5x10^9)^2))*(ln(.5))
→ −.38449=(t/((4.5x10^9)^2)*(−.69315)
→ .554701=t/((4.5x10^9)^2)
→ t = 2,498,044,246 years
Q20.3.10
Phenolphthalein is an indicator that turns pink under basic conditions. When an iron nail is placed in a gel that contains [Fe(CN)6]3−, the gel around the nail begins to turn pink. What is occurring? Write the half-reactions and then write the overall redox reaction.

Corrosion of a iron nail with a coiled copper wire, in agar-agar medium with ferroxyl indicator solution (potassium hexacyanoferrate(III), indicator of iron ions, and phenolphthalein, indicator of hydroxide ions). Image used with permission (CC BY-SA 3.0; Ricardo Maçãs).
Solution: Q20.3.10
When an iron nail is placed in a gel that contains [Fe(CN)6]3−, the gel around the nail begins to turn pink because the oxygen around the nail is being reduced when the nail is placed in the aqueous solution. The oxygen becomes OH- (hydroxide). Hydroxide is a very strong base. Since phenolphthalein is a basic indicator and hydroxide is a strong base, the phenolphthalein turns pink.
Iron in this reaction is oxidized (loses electrons): 3Fe2+(aq) + 2Fe(CN)63- (aq) → Fe3[Fe(CN)6]2 (s)
The reduction of oxygen is what turns the indicator pink: O2 (g) + 2H2O (l) + 4e- → 4 OH- (aq)
Q20.5.21
The reduction of Mn(VII) to Mn(s) by H2(g) proceeds in five steps that can be readily followed by changes in the color of the solution. Here is the redox chemistry:
- MnO4−(aq) + e− → MnO42−(aq); E° = +0.56 V (purple → dark green)
- MnO42−(aq) + 2e− + 4H+(aq) → MnO2(s); E° = +2.26 V (dark green → dark brown solid)
- MnO2(s) + e− + 4H+(aq) → Mn3+(aq); E° = +0.95 V (dark brown solid → red-violet)
- Mn3+(aq) + e− → Mn2+(aq); E° = +1.51 V (red-violet → pale pink)
- Mn2+(aq) + 2e− → Mn(s); E° = −1.18 V (pale pink → colorless)
- Is the reduction of MnO4− to Mn3+(aq) by H2(g) spontaneous under standard conditions? What is E°cell?
- Is the reduction of Mn3+(aq) to Mn(s) by H2(g) spontaneous under standard conditions? What is E°cell?
Solution: Q20.5.21
In order to solve this equation we must understand the Latimer diagram. The Latimer diagram shows the cell potential values for successive redox reactions shown in the diagram below. (The diagram is just an example and should not be followed for the questions below as the standard cell potentials are different).
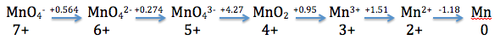
1. If we want to find out the standard cell potential for MnO4− to Mn3+(aq) by H2(g) we want to add the potentials from MnO4− to Mn3+(aq) and divide the sum by total number of electrons:
(0.564V) + (2.26V) + (.95) = 3.77V/ 4e-= 0.943V
Since this value is positive, we know that the reaction is spontaneous.
2. If we want to find out the standard cell potential for Mn3+(aq) to Mn(s) by H2(g) we want to add the potentials from Mn3+(aq) to Mn(s) and divide the sum by total number of electrons:
(1.51V) + (-1.18V) = 0.33V/ 3e-= 0.11V
Since this value is positive, we know that the reaction is spontaneous.

