Extra Credit 5
- Page ID
- 82964
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.5
Given the following pairs of balanced half-reactions, determine the balanced reaction for each pair of half-reactions in an acidic solution.
- Ca⟶Ca2++2e−,F2+2e−⟶2F\(\ce{Ca}⟶\ce{Ca}^{2+}+\ce{2e}^{-}\) , \(\ce{F}_2+\ce{2e}^{-}⟶\ce{2F}^{-}\)
- Ca⟶Ca2++2e-, F2+2e-⟶2F-
- ANSWER: F2+Ca⟶2F−+Ca2+
Steps To Solve:
- Make sure the charges on both the reactant and product side are balanced.
- reactant: Ca charge is 0 product: Ca2++2e- charge is 0
- reactant: F2+2e- charge is -2 product: 2F- is -2
-
Look at the coefficient of e- to check the charge of each chemical equation. Balance the reaction for e- to have the same coefficient.
-
Ca⟶Ca2++2e-
-
F2+2e-⟶2F-, e- has a coefficient of 2
-
Both e-'s have a coefficient of 2, so it's already balanced.
-
-
- Add two half reactions together.
-
F2+Ca+2e- ⟶ 2F-+Ca2++2e-
-
- cancel the e- and both side.
-
F2+Ca ⟶ 2F-+Ca2+
-
- Li⟶Li++e−,Cl2+2e−⟶2Cl−⟶Li++e-, Cl2+2e-⟶2Cl-
- ANSWER: 2Li+Cl2 ⟶ 2Li++2Cl-
Steps to Solve:
- Make sure the charges on the reactant and product sides are balance.
- reactant: Li charge is 0 product: Li++e- charge is 0
- reactant: Cl2+2e- charge is -2 product: 2Cl- is -2
-
Look at the coefficient of e- to check the charge of each chemical equation. Balance the reaction for e- to have the same coefficient.
-
-
Cl2 + 2 e- ⟶
2 Cl -
e-'s in each reaction have different coefficients, so we have to balance them by multiplying the Li reaction by two to get 2 as the coefficient of e-.
-
2 Li + +2 e
-
-
- Add reactant with reactant and product with product.
-
2Cl2Li+Cl2 +2e-⟶ 2Li++2Cl-+2e-
-
- cancel the e- and both side
-
2Cl2Li+Cl2 ⟶ 2Li++2Cl-
-
- Fe⟶Fe3++3e−,Br2+2e−⟶2Br−⟶Fe3++3e-, Br2+2e-⟶2Br-
- ANSWER: 3Br2+2Fe ⟶ 2Fe3++6Br-
- Steps to Solve:
- Make sure the charges on the reactant and product side are balanced.
-
reactant: Fe charge is 0 product: Fe3+ + 3 e- charge is 0
-
reactant: Br2 + 2 e-charge is -2 product: 2Br- is -2
-
-
Look at the coefficients of e- to check the charge of each chemical equation. Balance the reaction for e- to have the same coefficient.
-
-
Br2 + 2e- ⟶
2Br -, e- has a coefficient of 2-
Both have different coefficients, so we have to balance it by multiplying the Fe reaction by two, and multiplying the Br2 reaction by 3 to get 6 as the coefficient of e- for both of the reactions.
-
2 Fe 3+ +6 e- -
3Br2 + 6e- ⟶ 6
Br -
-
-
- Add the two reactions together
-
3Br2+2Fe+6e- ⟶ 2Fe3++6Br-+6e-
-
- Cancel the e- on both sides
-
3Br2+2Fe ⟶ 2Fe3++6Br
-
- ⟶Ag++e-, MnO4-+4H++3e-⟶MnO2+2H2O
-
ANSWER: MnO4-+4H++3Ag ⟶ 3Ag++MnO2+2H2O
-
- Steps to Solve:
- Make sure the charges on the reactant and product sides are balanced.
-
reactant: charge is 0 product: Ag++e- charge is 0
-
reactant: MnO4-+4H++3e-charge is 0 product: MnO2 + 2H2O charge is 0
-
-
Look at the coefficient of e- to check the charge of each chemical equation. Balance the reaction for both e-'s to have the same coefficient.
-
-
MnO4- + 4H+ + 3e- ⟶ MnO2+2H2O, e- has a coefficient of 3
-
Both have different coefficients, so we have to balance it by multiplying the Ag reaction by three to get the coefficient of 3 for e-
-
3 Ag
-
-
- Add two reactions together.
-
MnO4-+4H++3Ag+3e- ⟶ 3Ag++MnO2+2H2O+3e-
-
- Cancel the e-'s on both sides.
-
MnO4-+4H++3Ag ⟶ 3Ag++MnO2+2H2O
-
Q19.1.3
Write the electron configurations for each of the following elements and its 3+ ions:
- La and La3+
- ANSWERS: La= [Xe]6s25d1 | La3+= [Xe]
- Steps to Solve
- Look at the periodic table to find the orbital section of the element:
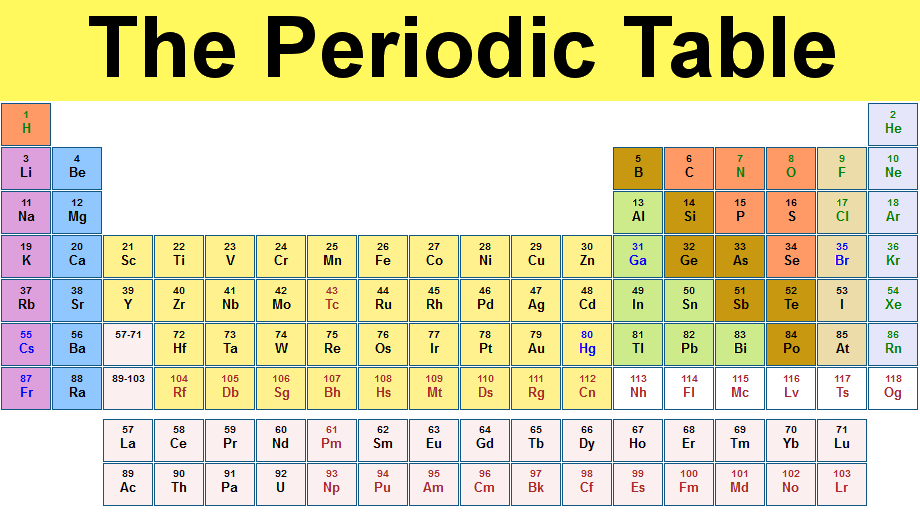
- La is in the d-block section
-
To write shorthand notation, you look at the noble gas that is on the period before that element with [] around it.
-
[Xe]
-
-
Add the other orbital sections between the noble gas and the element.
-
[Xe]6s25d1
-
-
-
To get La3+
-
Determine if the electron is gaining or losing by looking at the positive or the negative charge of the element. Positive means it is losing electrons, and negative means it is gaining electrons. Additionally, the number right next to the charge determines how many electrons is gained or lost.
-
3+ on top of La means the element is losing three electrons.
-
-
Determine the total number of electrons in the outer electron shell.
-
The total outer electron shell electron count for La is 3.
-
-
Subtract the number of outer electron shell electrons with the number of the charge.
-
There will be 0 outer electron shell left.
-
This mean that the shorthand notation for La3+ is [Xe].
-
-
- Sm and Sm3+
- ANSWERS: Sm= [Xe]6s24f6 | Sm3+= [Xe]4f5
- Steps to Solve:
- Look at the periodic table to find the orbital section of the element:

-
Sm is in the f-block section
-
To write shorthand notation, you look at the noble gas that is on the period before that element with [] around it.
-
[Xe]
-
-
Add the other orbital sections between the noble gas and the specified element.
-
[Xe]6s24f6
-
-
To get Sm3+
-
Determine if the electrons are gained or lost by looking at the positive or the negative charge of the element. Positive means the element has lost electrons, and negative means it has gained electrons. The number right next to the charge determines how many electrons are gained or lost.
-
3+ on top of Sm means the element is losing three electrons.
-
-
Determine the total number of electrons in the outer electron shell.
-
The total number of electrons in the outer electron shell for Sm is 8.
-
-
Subtract the number of outer electron shell electrons with the number of the charge.
-
There will be 5 outer electrons left.
-
-
To know which shell should be taken out first, need to determine which shell is higher in the orbitals
-
6s is a higher shell than 4f
-
This mean two electron is taken out from 6s and one is taken out from 4f which give the result of [Xe]4f5
-
-
- Lu and Lu3+
- ANSWERS: Lu= [Xe]6s24f145d1 | Lu3+= [Xe]4f14
- Steps to Solve:
- Look at the periodic table to find the orbital section of the element:

-
Lu is in the f-block section
-
To write shorthand notation, you look at the noble gas that is on the period before that element with [] around it.
-
[Xe]
-
-
Add the other orbital section that is between the noble gas and the element.
-
[Xe]6s24f145d1
-
-
To get Lu3+
-
Determine if the element is gaining or losing electrons by looking at the positive or the negative charge of the element. Positive means it losing electrons, and negative means it is gaining electrons. The number right next to the charge determines how many electrons are gained or lost.
-
3+ on top of Lu means the element is losing three electrons.
-
-
Determine the total number electrons in the outer electron shell.
-
The total outer electron shell electron count for Lu is 17.
-
-
Subtract the number of outer electron shell with the number of the charge.
-
There will be 14 outer electrons left.
-
-
To know which shell of electrons should be taken out first, determine which shell is higher in the orbitals.
-
higher 6s>5d>4f lower
-
This means two electron are taken out from 6s orbital and one is taken out from the 5d orbital which give the result [Xe]4f14
-
-
Q19.2.5
Draw diagrams for any cis, trans, and optical isomers that could exist for the following (en is ethylenediamine):
- Steps to Solve:
- Count how many numbers of Ligands there are in the nomenclature equation.
-
There are 4.
-
- Determine if the ligand is monodentate or polydentate. If it is polydentate, then determine how many bites they have (if it is bi ligand, tri ligand...)
-
en is polydentate; bi(2) ligand. NO2 and Cl2 is monodentate.
-
- Determine the coordination number.
-
It is 6.
-
- If the coordination number is 4 then there may be a cis and trans, if so determine them.
-
None; coordination number is 6
-
- Use the table to determine the optimal isomer.
-
Since en takes two bites that means there is total of 4 en. Therefore, it is not an optical isomer.
- [Co(en)2Cl2]+
-
ANSWER:
![FullSizeRender[4133].jpg](https://chem.libretexts.org/@api/deki/files/111462/FullSizeRender%255B4133%255D.jpg?revision=1&size=bestfit&width=320&height=301)
-
- Steps to Solve:
- Count how many numbers of Ligands there are in the nomenclature equation.
-
There are two.
-
- Determine if the ligand is monodentate or polydentate. If it is polydentate, then determine how many bite they have (if it is bi ligand, tri ligand...)
-
en is polydentate; bi(2) ligand. Cl is monodentate.
-
- Determine the coordination number.
-
It is 6.
-
- If the coordination number is 4 then there is a cis and trans, so determine them.
-
None. Coordination number is 6.
-
- Use the table to determine the optimal isomer.
- Since en takes two bites that means there is total of 4 en. Therefore, it is not optical isomer.
- [Pt(NH3)2Cl4]
- ANSWER:
![FullSizeRender[4134].jpg](https://chem.libretexts.org/@api/deki/files/111463/FullSizeRender%255B4134%255D.jpg?revision=1&size=bestfit&width=320&height=271)
- ANSWER:
- Steps to Solve:
- Count how many Ligands there are in the nomenclature equation.
-
There are two.
-
- Determine if the ligand is monodentate or polydentate. If it is polydentate, then determine how many bite they have (if it is bi ligand, tri ligand...)
-
Cl and NH3 are monodentate.
-
- Determine the coordination number.
-
It is 6.
-
- If the coordination number is 4 then there may be a cis and trans isomer.
-
None. Coordination number is 6.
-
- Use the table to determine the optimal isomer.
- Since there are 4 Cl, so it is not optical isomer.
- [Cr(en)3]3+
- ANSWER:
![FullSizeRender[4135].jpg](https://chem.libretexts.org/@api/deki/files/111464/FullSizeRender%255B4135%255D.jpg?revision=1&size=bestfit&width=320&height=284)
- ANSWER:
- Steps to Solve:
- Count how many numbers of Ligands there are in the nomenclature equation.
-
There is one.
-
- Determine if the ligand is monodentate or polydentate. If it is polydentate, then determine how many bite they have (if it is bi ligand, tri ligand...)
-
en is polydentate; bi ligand.
-
- Determine the coordination number.
-
It is 6.
-
- If the coordination number is 4 then there may be a cis and trans isomer.
-
None. Coordination number is 6.
-
- Use the table to determine the optimal isomer.
- Since en takes two bites that means there is total of 6 en. Therefore, it is not optical isomer.
- [Pt(NH3)2Cl2]
- ANSWER:
![FullSizeRender[4136].jpg](https://chem.libretexts.org/@api/deki/files/111465/FullSizeRender%255B4136%255D.jpg?revision=1&size=bestfit&width=550&height=292)
- ANSWER:
- Steps to Solve:
- Count how many Ligands there are in the nomenclature equation.
-
There are two.
-
- Determine if the ligand is monodentate or polydentate. If it is polydentate, then determine how many bite they have (if it is bi ligand, tri ligand...)
-
NH3 and Cl are monodetate.
-
- Determine the coordination number.
-
It is 4.
-
- If the coordination number is 4 then there may be a cis and trans isomer.
-
There is both cis and trans.
-
- Use the table to determine the optimal isomer.
- Since the complex compound is tetrahedral, it does not have optical isomers, but it does have cis and trans instead.
Q12.3.18
For the reaction
| [A] (M) | 0.230 | 0.356 | 0.557 |
| Rate (mol/L/s) | 4.17 × 10−4 | 9.99 × 10−4 | 2.44 × 10−3 |
- What is the order of the reaction with respect to [A], and what is the rate equation?
-
ANSWER: The rate equation is second order in A and is written as rate = k[A]2
-
- Steps to Solve:
Use the rate ratio of concentration equal to the rate of the reaction to determine the order of the concentration.
0.3560.2300.3560.230\((\frac{A2}{A1})\)x=\((\frac{Rate2}{Rate1})\)
\((\frac{0.356}{0.230})\)x=\((\frac{9.99\times10^{-4}}{4.17\times10^{-4}})\)
1.5478261x=2.3956835
x=2 This means that the rate of law is in 2nd order and would be written as rate=k[A]2
- What is the rate constant?
-
ANSWER: k = 7.88 × 10−3 L mol−1 s−1
-
- Steps to Solve:
Use the rate law equation you determined in the last problem to solve for the k constant
rate =k[A]2
4.17x10-4=k[0.230]2
4.17x10-4=k[0.0529]
7.88x10-3=k
Q12.6.9
Consider the reaction
CH4 + Cl2 → CH3Cl + HCl (occurs under light)
The mechanism is a chain reaction involving Cl atoms and CH3 radicals. Which of the following steps does not terminate this chain reaction?
1. CH3 + Cl → CH3CI
2. CH3 + HCl → CH4 + Cl
3. CH3 + CH3 → C2H2
4. Cl + Cl → Cl2
-
ANSWER: CH3 + HCl → CH4 + Cl
Steps to Solve:
Note that chain termination steps are the elementary steps of a chain reaction which use only radicals as reactants. Steps that use other reactants are not chain termination steps and therefore do not terminate this chain reaction.
- HCl is not a radical stated in the question and therefore makes the step CH3 + HCl → CH4 + Cl not a chain termination step
More about termination can be found here.
Q21.4.21
A sample of rock was found to contain 8.23 mg of rubidium-87 and 0.47 mg of strontium-87.
- Calculate the age of the rock if the half-life of the decay of rubidium by β emission is 4.7 × 1010 y
-
ANSWER: 3.8 billion years
-
Steps to Solve
- Find the he total mass (m) of the sample
-
m = 87Rb mg + 87Sr mg
-
=8.23 mg + 0.47 mg= 8.70 mg
- The weight fraction (f) of 87Rb is
-
f = 8.23 mg/8.70 mg =0.94597701
-
-
Assume the weight fraction of 87Rb at time 0 was 1. Hence,
-
f(t) = f(0)[(1/2)^(t/h)]
where f(t) is the weight fraction after t years. f(0) is the weight fraction at 0 years = 1. t is the time in years. h is the half life in years (4.7x1010)
-
0.94597701 = (1/2)(t/4.7x10)
ln(0.94597701) = (t/4.7x1010)ln(1/2)
t = (4.7x1010)ln(0.94597701)/ln(1/2)
t = 3.7657796x109
- If some \(^{87}_{38}Sr\) was initially present in the rock, would the rock be younger, older, or the same age as the age calculated in (a)? Explain your answer.
-
ANSWER: The rock would be younger than the age calculated in part (a). If Sr was originally in the rock, the amount produced by radioactive decay would equal the present amount minus the initial amount. This amount would be smaller than the amount used to calculate the age of the rock and the age is proportional to the amount of Sr, the rock would be younger.
-
Steps to Solve
Consider the amount of \(^{87}_{38}Sr\): if the amount is smaller then before, then it would be younger. If the amount is bigger, then it would be older. Therefore, according to the present amount of \(^{87}_{38}Sr\) in this rock, it will be younger.
Q20.3.9
The reaction Pb(s) + 2VO2+(aq) + 4H+(aq) → Pb2+(aq) + 2V3+(aq) + 2H2O(l) occurs spontaneously.
- Write the two half-reactions for this redox reaction
-
ANSWER: Pb(s)→Pb2+(aq)+2e-
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e-
Steps to Solve:
- Separate the elements or compounds into two reactions containing similar elements or compounds.
-
Pb(s)→Pb2+(aq)
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l) (All reactants and products here have been divided by the lowest common multiple of two for simplification)
-
-
Balance the charge on each side.
-
Pb(s)→Pb2+(aq)+2e-
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e-
-
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which reaction occurs at the cathode and which occurs at the anode?
-
ANSWER: Pb(s)→Pb2+(aq)+2e- occurs at anode
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e- occurs at cathode
Steps to Solve:
- Oil Rig is a pseudonym meaning if the element or compound in the reaction is losing electron(s), then the reaction is oxidizing; the element or compound will become more positively charged. If the element or compound in the reaction is gaining electron(s), then the reaction is reducing; the element or compound becomes more negatively charged.
-
Pb(s)→Pb2+(aq)+2e- is becoming more positive so it is oxidizing
- VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e- the element V is losing positive charge which means it becoming more negative, so the half reaction is reducing.
-
-
Oxidation is in the anode side and reduction is on the cathode side of the galvanic cell
-
Pb(s)→Pb2+(aq)+2e- anode
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e- cathode
-
- Which electrode is positively charged, and which is negatively charged?
-
ANSWER: Pb(s)→Pb2+(aq)+2e- is positive and VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e- is negative
-
Steps to Solve:
- Oil Rig is a pseudonym meaning if the element or compound in the reaction is losing electron(s), then the reaction is oxidizing; the element or compound will become more positively charged. If the element or compound in the reaction is gaining electron(s), then the reaction is reducing; the element or compound becomes more negatively charged.
-
Pb(s)→Pb2+(aq)+2e- is oxidizing so it is becoming more positive
-
VO2+(aq)+2H+(aq)→V3+(aq)+H2O(l)+e- is reducing, so the element V is losing positive charge and becoming more negative.
-
Q20.5.20
The standard electrode potential (E°) for the half-reaction Ni2+(aq) + 2e− → Ni(s) is −0.257 V. What pH is needed for this reaction to take place in the presence of 1.00 atm H2(g) as the reactant if [Ni2+] is 1.00 M?
- ANSWER: Anything less than 4.34
Steps to Solve:
- First, get the equation comprising the cell
- Half reactions are:
- Ni2+(aq) + 2e− → Ni(s) −0.257 V
- 2H+(aq) + 2e– → H2(g) +0.000 V
- [Ni2+] is the reactant so flip the Hydrogen reaction and combine the two reaction for the overall cell reaction. Plug in given molarities and pressures. The two electrons on either side are canceled out.
- Ni2+(aq, 1.00M) + H2(g, 1.00 atm) → Ni(s) + 2H+(aq, ? atm)
- Half reactions are:
- Next solve for E°cell. The anode is the more negative standard reduction potential.
- E°cell=E°cathode+E°anode
- = 0.257 V
- Next, use the Nernst Equation to solve for the amount of H+ needed to push E to greater than 0.
E=Eo−(0.0592/n)logQ
0<Eo−(0.0592/n)logQ
0<0.257-(0.0592/2)log([H+]2)
-0.257<-(0.0296)log([H+]2)
8.682>log([H+]2)
4.813E8>[H+]2
21939>[H+]
- Plug this concentration to convert it into pH
pH=log10[H+]
pH=log10(21939)
pH=4.34
- Less [H+] would drive the reaction forward. Decreasing [H+] decreases pH so anything less than 4.34 as a pH will drive the reaction forward.


![FullSizeRender[4132].jpg](https://chem.libretexts.org/@api/deki/files/111461/FullSizeRender%255B4132%255D.jpg?revision=1&size=bestfit&width=350&height=310)