Extra Credit 41
- Page ID
- 82955
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.1
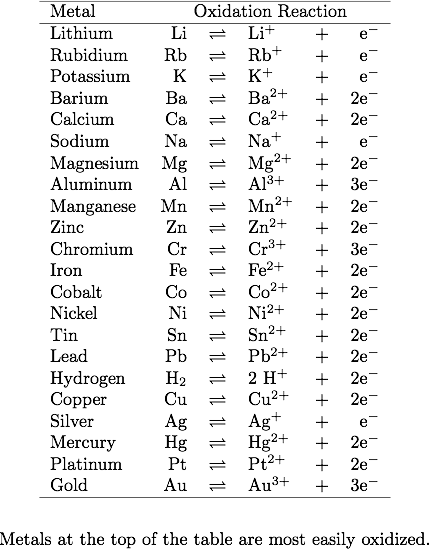
Which member of each pair of metals is more likely to corrode (oxidize)?
- Mg or Ca
- Au or Hg
- Fe or Zn
- Ag or Pt
Answer:
Corrosion is usually defined as the degradation of metals due to an electrochemical process. The more reactive a metal is, the more exergonic the reaction will be. Therefore, the reactive metals can be good reducing agents. To determine whether a metal is reactive, we need to look at its reduction potential. The reduction potential, E, is a measure of how much an element is willing to gain electrons (become reduced). A very positive E means that this element is more likely to gain electrons. The lower the potential is, the less reactive (oxidative) the metal is.
1. Calcium is more likely to oxidize because Ca is more reactive than Mg. Ca reacts strongly with water.
2. Hg is more easily oxidized because it is more reactive than Au. Just try to remember the activity series of metals.
3. Zinc is more easily oxidized than iron (-0.44V) because zinc has a lower reduction potential (-0.76V). Since zinc has a lower reduction potential, it is a more active metal.
4. Ag (standard reduction potential: +0.80V) is more reactive than Pt (+1.20V) so it is more easily oxidized.
-----------------------
Q12.3.4
How much and in what direction will each of the following affect the rate of the reaction: CO(g)+NO2(g) ⟶ CO2(g)+NO(g) if the rate law for the reaction is rate=k[NO2]2?
- Decreasing the pressure of NO2 from 0.50 atm to 0.250 atm.
- Increasing the concentration of CO from 0.01 M to 0.03 M.
Answer:
1. The rate of the reaction when the pressure of NO2 is 0.50 atm is:
rate1 = k [0.5]2 = 0.25k
The rate of the reaction when the pressure of NO2 is 0.250 atm is:
rate2 = k [0.25]2 = 0.0625k
rate1 : rate2 = 4, so the process reduces the rate by a factor of 4.
2. Since the rate law is only related to the pressure of NO2, the change in the concentration of CO does not affect the rate of the reaction.
-----------------------
Q12.5.13
Hydrogen iodide, HI, decomposes in the gas phase to produce hydrogen, H2, and iodine, I2. The value of the rate constant, k, for the reaction was measured at several different temperatures and the data are shown here:
| Temperature (K) | k (M−1 s−1) |
|---|---|
| 555 | 6.23 × 10−7 |
| 575 | 2.42 × 10−6 |
| 645 | 1.44 × 10−4 |
| 700 | 2.01 × 10−3 |
What is the value of the activation energy (in kJ/mol) for this reaction?
Answer:
To calculate the activation energy of a reaction, we use the Arrhenius equation (k=Ae−Ea/RT). We can get Ea= R ln (k2/k1) / (1/T1−1/T2)
where
Ea is the activation energy of the reaction in J/mol
R is the ideal gas constant = 8.3145 J/K·mol
T1 and T2 are temperatures in K
k1 and k2 are the reaction rate constants at T1 and T2
Therefore,
Ea = (8.3145) ln (6.23E-7 / 2.42E-6) / (1/575 - 1/555)
= 180027.6583 J/mol = 180 kJ/mol
-----------------------
Q21.4.8
The following nuclei do not lie in the band of stability. How would they be expected to decay? Explain your answer.
- 1534 P
- 23992 U --> 23490 Th +
- 3820 Ca
- 31 H
- 24594 Pu
Answer:
The graph of stable elements is commonly referred to as the Band (or Belt) of Stability. The graph consists of a y-axis labeled neutrons, an x-axis labeled protons, and a nuclei. At the higher end (upper right) of the band of stability lies the radionuclides that decay via alpha decay, below is positron emission or electron capture, above is beta emissions and elements beyond the atomic number of 83 are only unstable radioactive elements. Stable nuclei with atomic numbers up to about 20 have an neutron:proton ratio of about 1:1 (solid line).
- Alpha αα Decay: Alpha decay is located at the top of the plotted line, because the alpha decay decreases the mass number of the element to keep the isotope stable. This is accomplished by emitting a alpha particle, which is just a helium (HeHe) nucleus. In this decay pathway, the unstable isotope's proton number PP is decreased by 2 and its neutron (NN) number is decreased by 2. The means that the nucleon number A decreases by 4 (Equation 1).
- Beta β−β− Decay: Beta β−β− decay accepts protons so it changes the amount of protons and neutrons. the number of protons increase by 1 and the neutron number decreases by 1. This pathway occurs in unstable nuclides that have too many neutrons lie above the band of stability (blue isotopes in Figure 1).
- Positron β+β+ Decay: Positron β+β+ emission and electron capture is when the isotope gains more neutrons. Positron emission and electron capture are below the band of stability because the ratio of the isotope has more protons than neutrons, think of it as there are too few protons for the amount of neutrons and that is why it is below the band of stability (yellow isotopes in Figure 1).
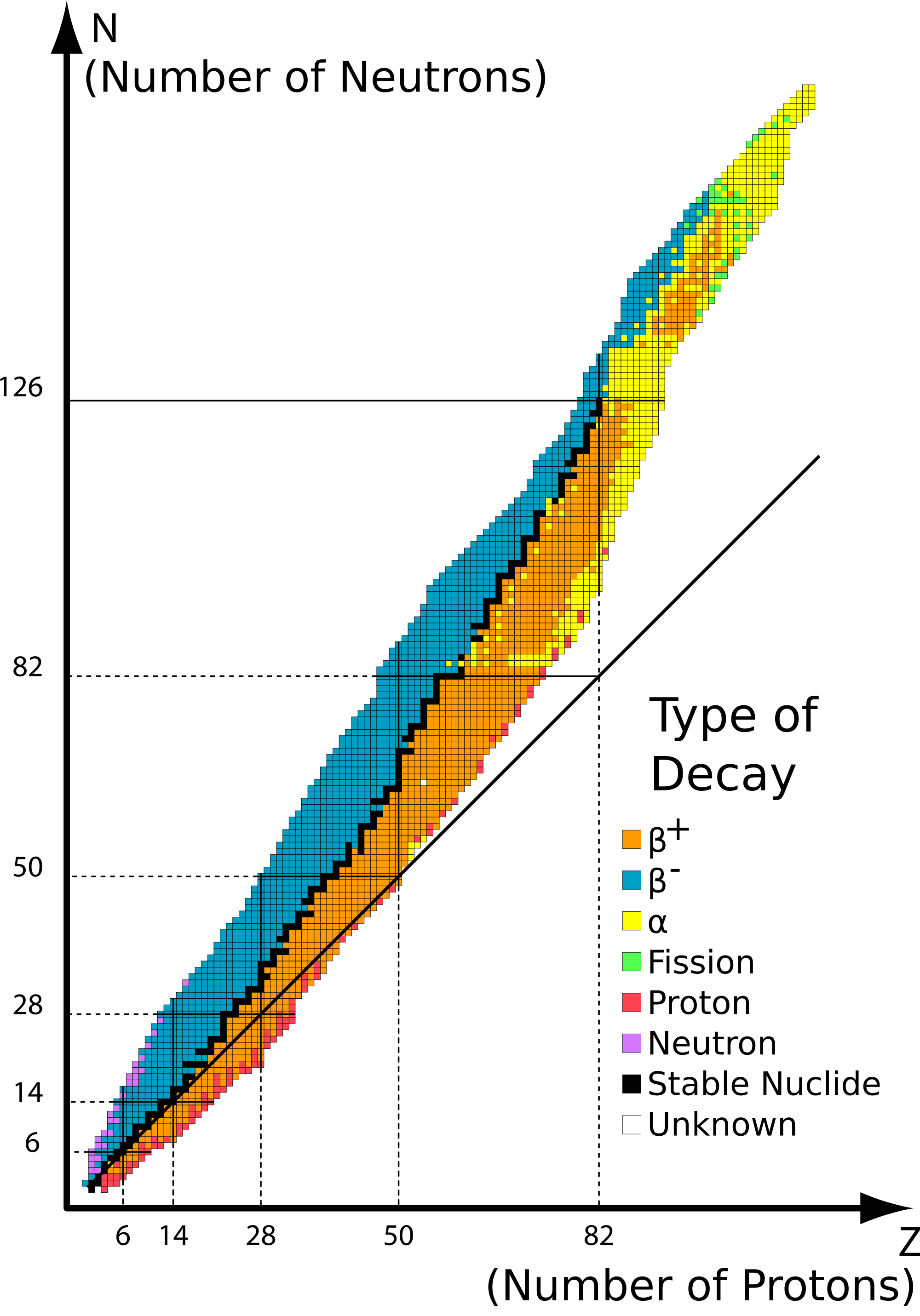
1. 3415 P (n/p=19/15>1, above the belt of stability so it yields ß- decay)
3415 P --> 3416 S + 0-1 e
2. 23992 U (protons = 92 > 83 so it yields α decay)
23992 U--> 23490 Th + 42 He + 10 n
3820 Ca (n/p=18/20<1, below the belt of stability so it yields ß+ emission)
3820 Ca --> 3818 Ar + 2 01 e
31 H (n/p=2/1>1, above the belt of stability so it yields ß- decay)
31 H --> 32 He + 0-1 e
24594 Pu (protons =94 > 83 so it yields α decay)
24594 Pu --> 24192 U + 42 He
-----------------------
Q20.2.12
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- A few drops of NiBr2 are dropped onto a piece of iron.
- A strip of zinc is placed into a solution of HCl.
- Copper is dipped into a solution of ZnCl2.
- A solution of silver nitrate is dropped onto an aluminum plate.
Answer:
1. The solution reacts with metal iron, and formed a little bit Ni metal on the surface of iron.
Ni2+ + 2e- --> Ni, Fe --> Fe3+ + 3e-
3Ni2+ + 6Br- + 2Fe --> 2Fe3+ + 3Ni + 6Br-
3Ni2+ + 2Fe --> 2Fe3+ + 3Ni
2. Zn reacts with HCl, forming bubbles which is hydrogen.
Zn --> Zn2+ + 2e-, 2H+ + e- --> H2
Zn + 2H+ + 2Cl- --> Zn2+ + H2 + 2Cl-
Zn + 2H+ --> Zn2+ + H2
3. no reaction, because Zn is more reactive than Cu
4. Silver is formed on the surface of aluminum.
Ag+ + e- --> Ag, Al --> Al3+ + 3e-
3Ag+ + 3NO3- + Al --> Al3+ + 3Ag + 3NO3-
3Ag+ + Al --> Al3+ + 3Ag
-----------------------
Q20.5.7
Occasionally, you will find high-quality electronic equipment that has its electronic components plated in gold. What is the advantage of this?
Answer:
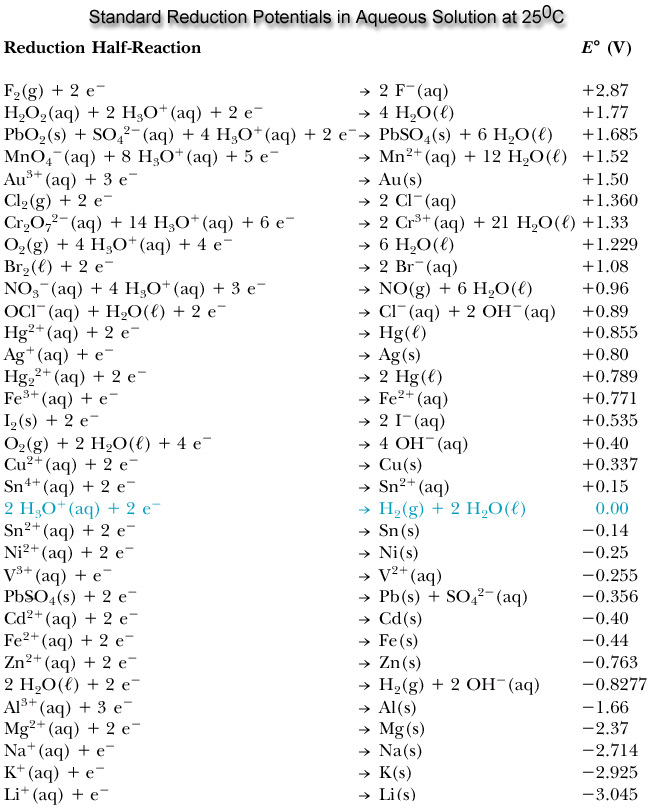
Gold is highly resistant to corrosion because of its very positive reduction potential.
-----------------------
Q24.6.3
Will the value of Δo increase or decrease if I− ligands are replaced by NO2− ligands? Why?
Answer:
The Δo is the crystal field splitting of a ligand.
The larger Δo corresponds with larger energy as well as stronger field splitting.
The value of Δo would increase because NO2− is a stronger ligand which means that it would have a larger split.
-----------------------
Q12.1
The following data were obtained for the reaction of methane with oxygen:
CH4(g)+2O2(g)→CO2(g)+2H2O(l)
|
time (min) |
[CH4](mol/L) |
[CO2] (mol/L) |
|---|---|---|
|
0 |
0.050 |
0 |
|
10 |
0.030 |
0.020 |
|
20 |
0.020 |
? |
|
30 |
0.015 |
? |
- How many moles of CO2 are produced for each mole of CH4 that is used up?
- What concentration of CH4 is used up after 10 minutes?
- What is the concentration of carbon dioxide produced after 20 minutes?
- Write an equation for reaction rate in terms of Δ[CO2] over a time interval.
- What is the reaction rate for the formation of carbon dioxide between 10 and 20 minutes?
- What is the average reaction rate between 0 and 30 minutes?
- Write an expression for reaction rate relating Δ[O2] to Δ[CO2].
- At what rate is O2 used up between 10 and 20 minutes?
Answer:
1. n (CO2) = n (CH4) = 1
2. 0.050 - 0.030 = 0.020 (mol/L)
3. [CO2 - produced] = [CH4 - used] = 0.050 - 0.020 = 0.030 (mol/L)
4. rate = Δ[CO2] / Δt
5. rate = [0.030 - 0.020] / [20 - 10] = 0.001 mol/L/min
6. [CO2 - 30 mins] = 0.050 - 0.015 = 0.035 (mol/L), rate = [0.035 - 0] / (30-0) = 0.001167 mol/L/min
7. rate = (- 1/2 Δ[O2]) / Δt = Δ[CO2] / Δt, so Δ[O2] = -2 Δ[CO2]
8. when rate = -1/2 Δ[O2] / Δt = - Δ[O2] / 20 = - [0.030x2 - 0.020x2] / 20 = - 0.001 mol/L/min

