Extra Credit 4
- Page ID
- 82953
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)17.1.4 Oxidation or Reduction?
a) Cl- -> Cl2
When determining whether a reaction is an oxidation or reduction reaction, it helps to remember the acronym OIL RIG (Oxidation is loss, Reduction is gaining). Because Cl- goes from a negative one oxidation state to a neutral charge (0) oxidation state, we can see that is loses electrons, making it an oxidation reaction.
b) Mn2+ -> MnO2
Referring back to OIL RIG, we can see that Mn2+ goes from a positive two oxidation state to a neutral charge (0) oxidation state, meaning it gains electrons. This is a reduction reaction because Mn2+ is being oxidized by the O2.
c) H2 -> H+
Because H is going from a neutral charge (0) oxidation state to a positive one charge oxidation state, it is losing an electron, making it an oxidation reaction.
d) NO3- -> NO
This is a reduction reaction because nitrogen goes from a +5 oxidation state to a +2 oxidation state, and so it gains electrons. The oxidation state of oxygen stays the same both in the reactants and products (-2 oxidation state).
19.1.2 Electron Configurations
a) Ti
For electron configurations, we have to look at the electron orbitals. The exponents represent how many electrons it takes to fill that orbital:
1s2
2s2 2p6
3s2 3p6 3d10
4s2 4p6 4d10 4f14
We have to follow this pyramid diagonally from right to left, although we sometimes fill the 3d orbital before the 4s orbital for stability. First determine how many electrons Ti has. Since it is number 22 on the periodic table, it has 22 electrons. So the electron configuration has to include all 22 electrons. It could look like this: Ti 1s2 2s2 2p6 3s2 3p6 4s2 3d2. But there is also a form of shorthand to use to make it less drawn out. We can use [Ar], which has 18 electrons rather than writing out the first 5 orbitals. We would then have [Ar] 4s2 3d2 instead to represent the electron configuration of Ti.
Another way to determine the electron figuration of an element or ion is by referring to a periodic table. First, locate the period (row) that Ti is in. We see that Ti, a transition metal, is located at the 4th period. Next, locate the group (column) that Ti is in. Below is the pattern of electron configurations on a periodic table. Using this table, we see that Ti has the electron configuration 1s22s22p63s23p64s23d2, or the shorthand [Ar] 4s2 3d2.


b) Ti2+
For Ti2+, it's similar to Ti, but it has two less electrons because of its 2+ charge. So we now have 20 electrons instead of 22. Using the shorthand, we can write it as [Ar] 3d2.
c) Ti3+
Ti3+ has one less electron than Ti2+ so we now have 19 electrons instead of 20. We can write its electron configuration as [Ar] 3d1.
d) Ti4+
Ti4+ has one less electron than Ti3+ so we now have 18 electrons instead of 19. Since it has the same amount of electrons as Ar, we can write the electron configuration of Ti4+ as simply [Ar].
19.2.4 Sketch the Structures
For each complex ion, first identify the number of ligands that the complex ion has. Then, determine the potential isomers that the complex ion may exhibit.
a. [Pt(H2O)2Br2] (square planar):
There are a total of 4 ligands ( two H2O and two Br2)
Because there are 2 of each ligands present and this is a square planar complex ion, we know that this complex ion exhibits cis/trans structure.
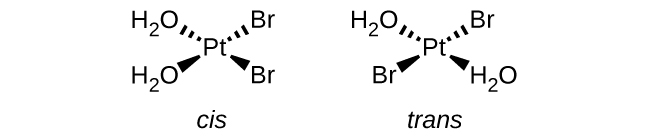
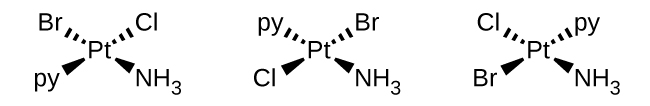
b. [Pt(NH3)(py)(Cl)(Br)] (square planar, py = pyridine, C5H5N):
There are a total of 4 ligands present (one NH3, one py, one Cl, and one Br). There are no geometric isomers seen because all 4 ligands are different. There are also no optical isomers because optical isomers are only possibly seen in octahedrals or tetrahedrals.
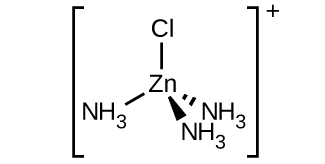
c. [Zn(NH3)3Cl]+ (tetrahedral):
There are 4 ligands present (three NH3 and one Cl).
A tetrahedral complex ion does not display any geometric isomers. There is also no optical isomers because there is a plane of symmetry.
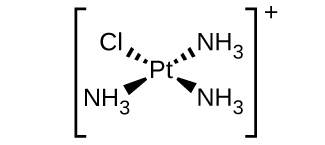
d. [Pt(NH3)3Cl]+ (square planar):
There are 4 ligands present (three NH3 and one Cl).
There are no geometric isomers for a square planar structure when it has three of the same ligands and a fourth other ligand. In order for a square planar structure to exhibit geometric isomers, it must have at least 1 pair of the same ligand to make is have cis/trans isomers. There is also no optical isomers because this complex ion has a plane of symmetry.
e. [Ni(H2O)4Cl2]:
There are 6 ligands present ( four H2O and two Cl), making it an octahedral structure.
Because a pair of ligands (Cl) is seen and this complex ion has a octahedral structure, we know that this complex ion has cis/trans geometric isomers. There are no optical isomers because there is a plane of symmetry present.
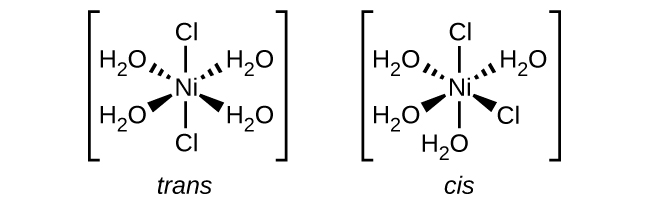
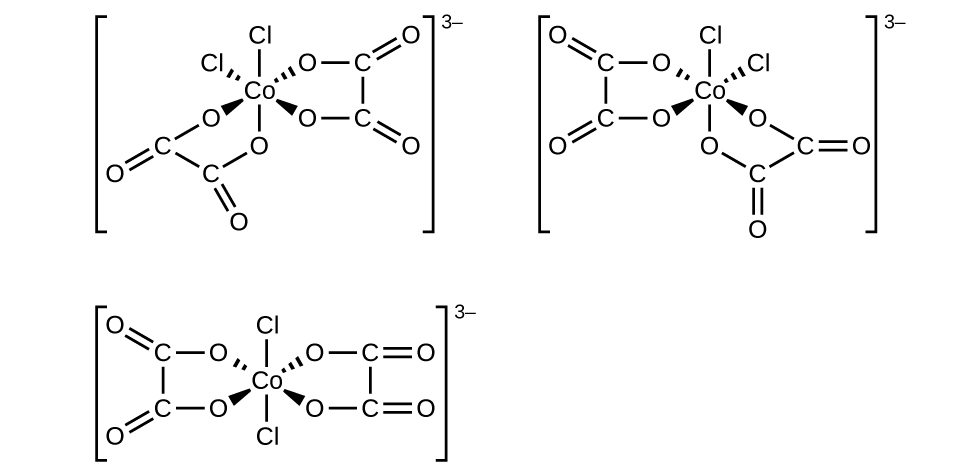
f. [Co(C2O4)2Cl2]3−:
There are 6 ligands present (two C2O4 is equivalent to four ligands because oxalate is a bidentate ligand and two Cl ligands.
This complex ion has a cis/trans geometric isomer because it is an octahedral and contains a pair of identical ligands, Cl. This complex ion has an optical isomer because there is no plane of symmetry and non-superimposable mirror images.
12.3.17 Given: H2(g) + 2NO(g) -> N2O(g) + H2O(g)
| [NO] M | 0.3 | 0.6 | 0.6 |
| [H2] M | 0.35 | 0.35 | 0.7 |
| Rate (mol/L/s) | 2.835x10-3 | 1.134x10-2 | 2.268x10-2 |
a) Determine the rate equation, the rate constant, and the orders with respect to each reactant.
The rate constant and the orders can be determined through the differential rate law. The general form of the differential rate law is given below:
aA + bB + cC ==> products
![]()
where A, B, and C are the concentrations of the reactants, k is the rate constant, and n,m, and p refer to the order of each reactant.
To find the orders of each reactant, we see that when [NO] doubles but [H2] doesn't change, the rate quadruples, meaning that [NO] is a second order reaction ([NO]2). When [H2] doubles but [NO] doesn't change, the rate doubles, meaning that [H2] is a first order reaction. So the rate law would look something like this:
Rate = k[NO]2[H2]
We can use this rate law to determine the value of the rate constant. Plug in the data for reactant concentration and rate from one of the trials to solve for k the rate constant. In this case, we chose to use the data from trial 1 from the second column of the data table.
2.835x10-3 = k[0.3]2[0.35]
k = .09 M-2/s-1
12.6.8 Given:
2NO + H2 -> N2 + H2O2 (slow)
H2O2 + H2 -> 2H2O (fast)
2NO + 2H2 -> N2 + 2H2O (overall chemical equation)
a) Determine the rate law of the given reaction.
It's important to remember that a reaction can only proceed as fast as its slowest elementary step. So the rate law for this reaction is going to be the exact rate law for the first half-reaction above, since it's the slow step and does not include any intermediates. Because this is an elementary step, we can use the coefficients of the reactants in the equation to represent the order of the reactants in the rate law, giving:
Rate = k[NO]2[H2]
21.4.20
What is the age of mummified primate skin that contains 8.25% of the original quantity of 14C?
The half-life of 14C is 5.73x103 years. We use the equation t1/2 = (ln2)/k to find the value of k because radioactive decay is a first order reaction:
5.73x103 = 0.693/k
k= 1.209424084 x 10-4
Now that we found k, we can use the first order reaction equation ln [A]/[A0]=-kt to solve for t, the time that has elapsed. Because [A0] is the initial concentration value, and [A] is the remaining concentration value after time t, we can say [A]=.0825[A0] because there is 8.25% of the initial concentration value remaining. So the equation before becomes:
ln (.0825[A0]/[A0]) = -kt (so the [A0]s will cancel out and it will just be ln .0825 on the left)
ln .0825 = -(1.209424084 x 10-4)t
t=20,629.29802=20,629 years old
20.3.8 Given: NO3-(aq) + H+(aq) + SO32-(aq) -> SO42-(aq) + HNO2
a) Write out the two half reactions.
First we must identify which species is being oxidized and which is being reduced by assigning oxidation numbers to each element. The nitrogen from NO3- on the reactants side has an oxidation number of +5 and the nitrogen from HNO2 in the products side has an oxidation number of +1; therefore, the species containing nitrogen is being reduced because nitrogen gains 2 electrons. The sulfur from SO32- on the reactants side has an oxidation number of +4 and the sulfur from SO42- on the products side has an oxidation number of +6; therefore, the species containing sulfur is being oxidized because sulfur loses 2 electrons.
Next, we write out the two half-reactions, one for oxidation and one for reduction.
Reduction: NO3-(aq) -> HNO2 (aq)
Oxidation: SO32-(aq) -> SO42-(aq)
Then we balance each of these half reactions by first balancing all of the elements present in the half reactions except for hydrogen and oxygen. Next, add H2O to balance out the oxygens. Then use H+ to balance out the hydrogens. Lastly, we must balance the charges of each half reaction by adding the appropriate number of electrons, resulting in our 2 balanced half reactions.
Reduction: NO3-(aq) + 3H+ + 2e- -> HNO2(aq) + H2O(l)
Oxidation: SO32-(aq) + H2O(l) -> SO42-(aq) + 2H+ + 2e-
b) If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which electrode corresponds to which half-reaction?
Oxidation always occurs at the anode and reduction occurs at the cathode. Because NO3- reaction is being reduced, its half-reaction would correspond to the cathode electrode. The SO32- reaction is being oxidized, so that half-reaction would correspond to the anode electrode.
c) Which electrode is negatively charged, and which is positively charged?
The anode is negatively charged and the cathode is positively charged.
20.5.19
Potentiometric titrations are an efficient method for determining the endpoint of a redox titration. In such a titration, the potential of the solution is monitored as measured volumes of an oxidant or a reductant are added. Data for a typical titration, the potentiometric titration of Fe(II) with a 0.1 M solution of Ce(IV), are given in the following table. The starting potential has been arbitrarily set equal to zero because it is the change in potential with the addition of the oxidant that is important.
| Titrant (mL) | E (mV) |
| 2.00 | 50 |
| 6.00 | 100 |
| 9.00 | 255 |
| 10.00 | 960 |
| 11.00 | 1325 |
| 12.00 | 1625 |
| 14.00 | 1875 |
a) Write the balanced chemical equation for the oxidation of Fe2+ by Ce4+.
Because Fe2+ is oxidized, it loses an electron to Ce4+, which is reduced because it gains an electron.
Ce4+(aq) + Fe2+(aq) -> Ce3+(aq) + Fe3+(aq)
b) Plot the data and then locate the endpoint.

The endpoint would be at about 10 mL and 1000 mV because that is when the line is the steepest. The endpoint is found when the moles of titrant equals the moles of the anayte.
c) How many millimoles of Fe2+ did the solution being titrated originally contain?
Given that the original concentration of the titrant is 0.1M and it took 10 mL to reach the end point, you can multiply the two together to see how many millimoles of Fe2+ were in the solution being titrated. You find that there was 1 millimole.
(1 mol/liter) x (10mL)= 1mmol

