Extra Credit 38
- Page ID
- 82951
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.5.6
Why do batteries go dead, but fuel cells do not?
S 17.5.6
-Secondary batteries go dead because as you recharge them, an undesired reaction happens. This corrosion (unwanted oxidation reaction) when charging a battery causes Fe2O3 . H2O to build up. Since batteries are self contained with a limited amount of reagents, this build up will "kill" them because the undesired product inhibits the reaction that creates voltage.
-Fuel cells do not go bad simply because they are not self contained. They are commonly called flow batteries, because a constant flow of reagents must be provided. So long as the reagents are provided, the cell can continue to create power. The cell still undergoes an electrochemical reaction, however instead of the products (desired as well as undesired) being self contained, they are released.
Q 12.3.1
How do the rate of a reaction and its rate constant differ?
S 12.3.1
Reaction rate is merely how fast a reaction proceeds in a given amount of time. The rate constant basically takes into account the factors the would influence the rate. For example: temperature, pressure, and catalysts will affect the rate constant. This change in constant, then affects the reaction rate: (constant)(reactants)=(rate) i.e. an increase in rate constant will increase the rate. Basically, the rate reacts to environmental, concentration, and other effects whereas the reaction constant once determined experimentally.
Q 12.5.10
The rate constant for the decomposition of acetaldehyde, CH3CHO, to methane, CH4, and carbon monoxide, CO, in the gas phase is 1.1 × 10−2 L/mol/s at 703 K and 4.95 L/mol/s at 865 K. Determine the activation energy for this decomposition.
S 12.5.10
To solve this, you're going to have to use the Arrhenius equation which is: 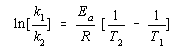 .
.
Since we are solving for Ea, you will rearrange the equation to
You then assign each duo to k1 and t1, and then k2 and t2. I decided k1 would be .011 and t1 would be 703k (note: order of these doesn't matter. Either can be k1 and t1 as long as you keep the rate and its temperature together)
Then you plug in the values:
Divide by 1000:
Final Answer:
Q 21.4.5
Why is electron capture accompanied by the emission of an X-ray?
S 21.4.5
Why is electron capture accompanied by the emission of an X-ray?
Electrons are captured from the 1s orbital most often. This then leaves a void. In order to fill this spot, an electron from a higher energy shell must fall down to the 1st level. The energy lost from the electron going from a higher energy state to a lower one is then emitted as an x ray.
Q 20.2.9
Balance each redox reaction under the conditions indicated.
- CuS(s) + NO3−(aq) → Cu2+(aq) + SO42−(aq) + NO(g); acidic solution
- Ag(s) + HS−(aq) + CrO42−(aq) → Ag2S(s) + Cr(OH)3(s); basic solution
- Zn(s) + H2O(l) → Zn2+(aq) + H2(g); acidic solution
- O2(g) + Sb(s) → H2O2(aq) + SbO2−(aq); basic solution
- UO22+(aq) + Te(s) → U4+(aq) + TeO42−(aq); acidic solution
S 20.2.9
Procedure for balancing redox reactions:
1) Split up into half reactions by assigning oxidation numbers. The element that becomes more positive is oxidized, and the one that becomes more negative is oxidized.
2) Balance the core element that is ox/red.
3) Balance oxygen by adding water to the appropriate side.
4) Balance hydrogen by adding H+
5) Balance the charge by adding electrons.
6) Multiply each half reaction by the appropriate coefficient, to ensure both have the same number of electrons. After, combine the two, and cancel the species that occur on both sides.
FOR BASIC:
Procedure is the same, however you add an OH- per H+ on both sides.
These steps shown below in action:
a)CuS(s) + NO3-(aq) → Cu2+(aq) + SO42-(aq) + NO(g); acidic solution
In this redox reaction, the S's oxidation state goes from - to +, so it is being oxidized.
Oxidation: CuS -> Cu2++ SO42-
Add water: 4H2O + CuS -> Cu2+ + SO42-
Add protons: 4H2O+CuS -> Cu2++SO42- + 8H+
Add Electrons: 4H2O + CuS-> Cu2++SO42-+8H+ + 8e-
As well as, N goes from +5 to +2, so it is reduced.
Reduction: NO3- -> NO
Add water: NO3- -> NO + 2H2O
Add protons: 4H++NO3- -> NO +2H2O
Add Electrons: 3e- +4H++NO3- -> NO + 2H2O
Then, multiply both equations by a common factor due to both having different electrons which will give you this result.
Result: 8H++ 3CuS(s) + 8NO3-(aq) -> 8NO (g) + 3 Cu2+(aq) +3SO42-(aq) +4H2O (l)
b) Ag(s) + HS-(aq) + CrO42-(aq) → Ag2S(s) + Cr(OH)3(s); basic solution
Oxidation: 3(HS-+2Ag->Ag2S+H++2e-)
Reduction: 2(3e-+5H++CrO42-->Cr(OH)3+H2O)
Overall (in basic): 3HS-(aq)+6Ag(s)+5H2O(l)-> 3Ag2S(s) + 7OH-+2Cr(OH)3(s)
c)Zn(s) + H2O(l) → Zn2+(aq) + H2(g); acidic solution
Oxidation: Zn -> Zn2+ + 2e-
Reduction: 2e-+2H++H2O -> H2 + H2O
Overall: Zn(s)+2H+-> Zn2+(aq)+H2(g)
d)O2(g) + Sb(s) → H2O2(aq) + SbO2-(aq); basic solution
Oxidation: 2(2H2O+Sb -> SbO2- +4H++3e-)
Reduction: 3(2e-+2H++O2 -> H2O2)
Overall: 2OH-+2H2O(l)+2Sb(s)+3O2(g) -> 2SbO2-(aq) +3H2O2(aq)
e)UO22+(aq) + Te(s) → U4+(aq) + TeO42-(aq); acidic solution
Oxidation: 4H2O+Te -> TeO42-+8H++6e-
Reduction: 3(2e-+4H++UO22+-> U4++2H2O)
Overall: 4H++ Te(s) +3UO22+(aq)->TeO42-(aq)+2H2O(l)+3U4+(aq)
Q 20.5.4
For any spontaneous redox reaction, E is positive. Use thermodynamic arguments to explain why this is true.
S 20.5.4
For this, you have to remember the thermodynamic triangle. The point of an electrochemical cell (where redox reactions occur) is to do work in the form of electrical energy, therefore, positive voltage (from the spontaneous reaction) will generate work. Furthermore, negative delta G will result in a spontaneous process because reactants have more energy than the products in this case. For this rule to stay consistent in the equation ΔG=-nFEcell , E MUST be positive.
Q 24.5.1
- How many unpaired electrons are found in oxygen atoms ?
- How many unpaired electrons are found in bromine atoms?
- Indicate whether boron atoms are paramagnetic or diamagnetic.
- Indicate whether F- ions are paramagnetic or diamagnetic.
- Indicate whether Fe2+ ions are paramagnetic or diamagnetic.
S 24.5.1
a)How many unpaired electrons are found in oxygen atoms ?
O has an electron configuration of [He]2s22p4. Since the P orbital can hold a total of 6 electrons, there are 2 unpaired electron in an oxygen atom. This also means O is a paramagnetic ion due it not having an equal amount of paired electrons.
b)How many unpaired electrons are found in bromine atoms?
Br has an electron configuration of [Ar]4s23d104p5. Since the P orbital can hold 6 electrons, Br has 1 unpaired. This also means Br is a paramagnetic ion due it not having an equal amount of paired electrons.
c)Indicate whether boron atoms are paramagnetic or diamagnetic.
Boron has an electron configuration of [He]2s22p1. Since there are unpaired electrons (the p shell isn't completely full with 6 electrons), B is paramagnetic. A paramagnetic ion contains an unequal amount of electrons in an electron orbital.
d)Indicate whether F- ions are paramagnetic or diamagnetic.
F- means that F has one extra electron. That gives it the same configuration as Neon, shown:  (credit: Libretext) Since every shell is completely full, this is a diamagnetic ion. A Diamagnetic ion contains an equal paring of electrons in an electron orbital. F- has an excited electron configuration of [He] 2s24p6.
(credit: Libretext) Since every shell is completely full, this is a diamagnetic ion. A Diamagnetic ion contains an equal paring of electrons in an electron orbital. F- has an excited electron configuration of [He] 2s24p6.
e)Indicate whether Fe2+ ions are paramagnetic or diamagnetic.
Fe2+ means it has two less electrons, giving it a configuration of: [Ar] 3d6 (because transition metal ions pull from the 2 sub-orbital first). Since the d orbital holds 10 electrons, and there are only 6 (leaving a partially full subshell) - it is paramagnetic. A paramagnetic ion contains an unequal amount of electrons in an electron orbital.
Q 14.7.8
The text identifies several factors that limit the industrial applications of enzymes. Still, there is keen interest in understanding how enzymes work for designing catalysts for industrial applications. Why?
S 14.7.8
There is such a keen interest in understanding how enzymes work because even though they have limited use in industrial applications, they work phenomenally in the body. Enzymes that work as catalysts allow the body to work efficiently and lower activation energy in almost all processes. If this behavior could be mimicked in an industrial setting, energy, time, and labor could be saved.

