Extra Credit 33
- Page ID
- 82946
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.5.1
What are the desirable qualities of an electric battery?
S17.5.1
Desirable qualities of an electric battery are batteries that contain at least two or more electrochemical cells, that can be converted into either chemical or electrical energy used to produce current. They should also be heat resistant as well as containing unlimited voltage and have a high current output.
Desirable qualities of an electric batter are ones that can be self contained and store energy for later usage. Important things to note are that they can contain multiple voltaic cells to increase the output of the battery and are super convenient since they are portable.
Q12.1.6
Consider the following reaction in aqueous solution:
\(5Br^−(aq)+BrO_3^{−}(aq)+6H^+(aq) \to 3Br^2(aq)+3H_2O(l) \)
If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4 M s−1, what is the rate of appearance of Br2(aq) at that moment?
S12.1.6
1. Define the rate of the reaction:
*remember the general reaction is aA + bB → cC+ dD
\(rate =- \frac{\Delta[A]}{a\Delta{t}}=- \frac{\Delta[B]}{b\Delta{t}}= \frac{\Delta[C]}{c\Delta{t}}=\frac{\Delta[D]}{d\Delta{t}}\)
Therefore when using the rate disappearance of Br-(aq), the reaction rate turns out to be:
\(rate =- \frac{\Delta[Br^-]}{5\Delta{t}}=- \frac{\Delta[BrO^-_3]}{\Delta{t}}= -\frac{\Delta[H^+]}{6\Delta{t}}=\frac{\Delta[Br_2]}{3\Delta{t}}=\frac{H_2O}{3\Delta{t}}\)
2. Set the rates equal to each other:
\(rate =- \frac{\Delta[Br^-]}{5\Delta{t}}= \frac{\Delta[Br_2]}{3\Delta{t}}\)
You then get this as your answer, \(-\frac{\Delta[Br^-]}{\Delta{t}}= -3.5x10^{-4} Ms^{-1}\)
3. Solve the equation:
\(\frac{(3.5x10^{-4})(3)}{5} = \frac{\Delta[Br_2]}{\Delta{t}}\)
\(\frac{\Delta[Br_2]}{\Delta{t}} = 2.1 x 10^{-4} Ms^{-1}\)
Q12.5.5
Describe how graphical methods can be used to determine the activation energy of a reaction from a series of data that includes the rate of reaction at varying temperatures.
S12.5.5
The graphical methods that determine activation energy is determined by the Arrhenius equations that shows change in temperature and the effects it has on a rate constant.
\(k = Ae^\frac{-E_a}{RT}\)
\(ln \left(\frac{k_1}{k_2}\right) = \left(\frac{-E_a}{R}\right)\left(\frac{1}{T_1} - \frac{1}{T_2}\right)\)
After plotting ln k versus 1/T, you will find the slope's value to be \( \frac{-E_a}{R}\) in which you can just multiply by the constant R (8.3145 J/(mol*K) to determine the activation energy.
Q21.3.8
For the reaction \( \ce{^14_6}C \to \ce{^14_7}N+ ? \), if 100.0 g of carbon reacts, what volume of nitrogen gas (N2) is produced at 273 K and 1 atm?
S21.3.8
1. With Stoichoiometry, convert the mass of carbon into moles of nitrogen
\((100g\;C)(\frac{1\;mol\;C}{12g\;C})(\frac{1\;mol\;N_2}{1\;mol\;C}) = 8.33\;moles\;of\;N_2\)
2. Plug in the givens from the problem and solve for unknown variable:
\((1\;atm)(V) = (8.33\;moles\;of\;N_2)(0.08206\frac{L\cdot atm}{mol \cdot K})(273\;K)\)
V = 186.6 L of \(N_2\) gas
Since the reaction is at STP, the moles of \(N_2\) can be converted using the ratio of 1 mole: 22.4 L
Using this ratio, you'll get that $$ 8.33\;moles\;of\;N_2 \times \frac{22.4 L}{1 mol N_2}$$
$$=186.6 L $$
Q20.2.4
Single-displacement reactions are a subset of redox reactions. In this subset, what is oxidized and what is reduced? Give an example of a redox reaction that is not a single-displacement reaction.
S20.2.4
There are many ways to decipher what is being oxidized and what is being reduced in a redox reaction, but the most known way to decide between the two is the phrase, "oil rig" which stands for "oxidation is lose and reduction is gain". You compare the number of electrons that are loss and gained within the reaction.
An example of a single displacement reaction is:
\(2Fe + 6HCl \rightarrow 2FeCl_3 + 3H_2\)
Although redox reactions can not have single displacement reactions, they are able to have combustion, decomposition combination and disproportionation reactions.
An example of a redox reaction that is not a single displacement reaction is:
\(C_3H_8 + 5O_2 \rightarrow 3CO_2 + H_2O + \) light and heat
Q20.4.23
If you place Zn-coated (galvanized) tacks in a glass and add an aqueous solution of iodine, the brown color of the iodine solution fades to a pale yellow. What has happened? Write the two half-reactions and the overall balanced chemical equation for this reaction. What is E°cell?
S20.4.23
The brown color turning into a pale yellow tells us the that iodine is being reduced by zinc.
1. Using the standard reduction potential chart that is usually given, find the two half reactions and look for the it's cell potential on the standard reduction chart
\(Zn^{2+}(aq) + 2e^- \to Zn(s)\quad E^o=-0.76\)
\(I_2(s) + 2e^- \to 2I^-(aq)\quad E^o = 0.54\)
2. Since zinc is being oxidized, it loses electrons. Therefore flip the equation for zinc along with the sign of the its standard cell potential.
\(Zn(s) \to Zn^{2+}(aq) + 2e^-\quad E^o = 0.76\)
\(I_2(s) + 2e^- \to 2I^- (aq) \quad E^o=0.54\)
3. Cancel out the \(2e^-\) and form the overall equation
\(I_2(s) + Zn(s) \to 2I^-(aq) + Zn^{2+}(aq)\)
4. Use this equation to find the \(E^o_{cell}\).
\(E^o_{cell} = E^o_{cathode} - E^o_{anode}\)
\(E^o_{cell} = 0.54 - (-0.76) = 1.30\;V\)
\(E^o_{cell} = 1.30\;V\)
You may also find the cell potential by adding the half reaction that is oxidized the half reaction that is reduced: 0.76 V + 0.54 V = 1.30 V
Q20.9.8
What mass of copper metal is deposited if a 5.12 A current is passed through a Cu(NO3)2 solution for 1.5 h.
S20.9.8
1. Look up half reaction of copper from standard reduction table
\(Cu^{2+} + 2e^- \rightarrow Cu\)
2. Solve for the mass of copper
\( (\frac{5.12 C}{s}) \times (1.5hr \times \left(\frac{3600s}{1hr}\right) \times \left(\frac{1 mol\,e^-}{96485 C}\right) \times \left(\frac{1 mol Cu(NO_3)_2}{2 mol\,e^-}\right) \times \left(\frac{1molCu}{1molCu(NO_3)_2}\right) \times \left(\frac{63.546gCu}{1 mol Cu}\right) = 9.105g\,of \,Cu\)
Note: 1 Amp = 1 coulomb/sec
Q14.6.6
Cyclopropane, a mild anesthetic, rearranges to propylene via a collision that produces and destroys an energized species. The important steps in this rearrangement are as follows:
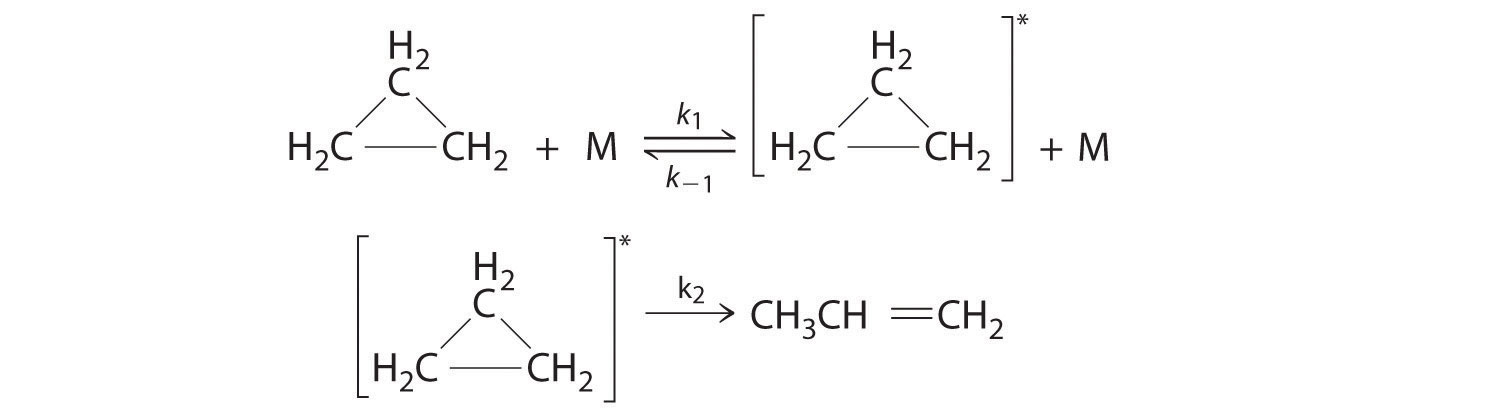
where M is any molecule, including cyclopropane. Only those cyclopropane molecules with sufficient energy (denoted with an asterisk) can rearrange to propylene. Which step determines the rate constant of the overall reaction?
S14.6.6
When searching for the rate limiting step, that can be easily found because it is generally the slowest step in the reaction. Also the k2 step is likely to be rate limiting; the rate cannot proceed any faster than the second step.

