Extra Credit 29
- Page ID
- 82941
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.2
Question:
For the ΔG° values given here, determine the standard cell potential for the cell.
- 12 kJ/mol, n = 3
- −45 kJ/mol, n = 1
Solution:
ΔG° stands for the free energy of a reaction at standard state conditions where the partial pressures of any gases involved in the reaction is 0.1 M Pa the concentrations of all aqueous solutions are 1 M. ΔG° is also a measure of the amount of useful work that can be obtained from a thermodynamic system under constant pressure and temperature.
The standard cell potential Eocell is the cell potential obtained when all of the reactants and products are in their standard state.
The relationship between ΔGo and Eo is given by the following equation:
Go=-nFEo
Where “n” is the number of moles of electrons according to a balanced oxidation-reduction reaction and “F” is Faraday’s constant 96,485 J/(Vmol). The negative sign seen in this equation shows that work had been done by the system.
-
If E°cell > 0, then the process is spontaneous
-
If E°cell < 0, then the process is non-spontaneous
In order for us to calculate the standard cell potential, the equation seen above can be rearranged as follows:
$$E°_{cell}=\frac{-ΔG°}{nF}$$
a) n=3, ΔG°=12kJ/mol, F=96,485 J/(V•mol)
$$E°_{cell}=\frac{-12kJ}{(3mol)(96,485\frac{J}{V\cdot mol})}\cdot \frac{10^{3}J}{1kJ}=\frac{-12\cancel{kJ}}{(3\cancel{mol})(96,485\frac{\cancel{J}}{V\cdot \cancel{mol}})}\cdot \frac{10^{3}\cancel{J}}{1\cancel{kJ}}=-0.0414V$$
b) n=1, ΔG°=-45kJ/mol, F=96,485 J/(Vmol)
$$E°_{cell}=-\frac{-45kJ}{(1mol)(96,485\frac{J}{V\cdot mol})}\cdot \frac{10^{3}J}{1kJ}=\frac{45\cancel{kJ}}{(1\cancel{mol})(96,485\frac{\cancel{J}}{V\cdot \cancel{mol}})}\cdot \frac{10^{3}\cancel{J}}{1\cancel{kJ}}=0.466V$$
Q12.1.2
Question
Ozone decomposes to oxygen according to the equation 2O3(g)⟶3O2(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen.
Solution
General balanced equation:
$$aA+bB \rightarrow cC + dD$$
Equation showing the rate of formation of C and D and rate of disappearance of A and B:
$$- \dfrac{1}{a}\dfrac{\Delta [A]}{\Delta t} = - \dfrac{1}{b}\dfrac{\Delta [B]}{\Delta t} = \dfrac{1}{c}\dfrac{\Delta [C]}{\Delta t} = \dfrac{1}{d}\dfrac{\Delta [D]}{\Delta t}$$
where: [ ]=concentration t=time a,b,c,d=moles, coefficients in the balanced equation.
The given balanced chemical equation shows that 2 moles of ozone (O3) decomposes for every 3 moles of oxygen gas (O2) that is produced. Because ozone is "disappearing" as the reaction goes on, we will use a negative sign to indicate this property. Because oxygen gas is being produced, its rate of reaction will be preceded by a positive sign. The rate of the reaction of the decomposition of ozone into the formation of oxygen gas is represented as:
$$Rate=-\frac{Δ[O3]}{2ΔT}=\frac{Δ[O2]}{3ΔT}$$
Q12.4.20
Question:
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene (CH2=CH–CH=CH2) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
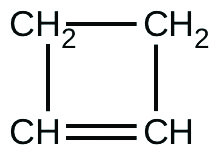
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 × 10−4 s−1 at 150 °C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.
Solution:
Step 1: The integrated rate law for a first-order reaction is:
$$[A_{t}]=[A_{0}]e^{-kt}$$
where [A0]=the initial concentration of reactant A k=the first order rate constant \([A_{t}]\)=the concentration of reactant A at time "t" t=time
Step 2: Plug-in t=30 minutes, k=2.0 × 10−4 s−1, and [A0]=55 torr into the integrated rate law equation for a first order reaction to solve for the partial pressure of cyclobutene after 30 minutes:
$$[A_{30}]=(55 torr)e^{-(2.0x10^{-4}\frac{1}{sec})(30min\cdot\frac{60sec}{1 min})}$$
$$[A_{30}]=(55 torr)e^{-(2.0x10^{-4}\frac{1}{\cancel{sec}})(1800\cancel{sec})}$$
$$[A_{30}]=(55 torr)e^{-0.36}$$
$$[A_{30}]=(55 torr)e^{-0.36}$$
Step 3: To find the initial concentration of cyclobutene , use the ideal gas equation:
\(PV=nRT\) and rearrange to get \(n= \frac{PV}{RT}\)
Plug-in the following values into the ideal gas equation to find the initial moles of cyclobutene
\(P=\cancel{torr}\cdot\frac{1atm}{760\cancel{torr}}=0.07237atm\) V=0.53 L T=150°C (423.15 K) R= 0.08206\(\frac{L*atm}{mol*K}\)
$$n=\frac{(55torr)(0.53L)}{(0.08206\frac{L*atm}{mol*K})(423.15K)}$$
$$n=\frac{(0.07237\cancel{atm})(0.53\cancel{L})}{(0.08206\frac{\cancel{L}*\cancel{atm}}{mol*\cancel{K}})(423.15\cancel{K})}=0.00110\>moles\>of\>cyclobutene\>initially$$
Step 4: Find the initial concentration (M) of cyclobutene
$$[A_{0}]=\frac{n}{V}=\frac{0.00110 moles}{0.53 L}=0.00208 M$$
Step 5: Use the integrated rate law equation for a first order reaction to find the concentration of cyclobutene after 30 minutes.
$$[A_{t}]=[A_{0}]e^{=kt}$$
$$[A_{30}]=(0.00208M)e^{-(2.0x10^{-4}\frac{1}{sec})(30min\cdot\frac{60sec}{1 min})}$$
$$[A_{30}]=(0.00208M)e^{-(2.0x10^{-4}\frac{1}{\cancel{sec}})(1800\cancel{sec})}$$
$$[A_{30}]=(0.00208M)e^{-0.36}= 0.00145M$$
Q21.3.4
Question:
Complete each of the following equations:
a) \(^{7}_{3}Li+?\rightarrow2^{4}_{2}He\)
b) \(^{14}_{6}C\rightarrow^{14}_{7}N+?\)
c) \(^{27}_{13}Al+^{4}_{2}He\rightarrow?+^{1}_{0}n\)
d) \(^{250}_{96}Cm\rightarrow?+^{98}_{38}Sr+4^{1}_{0}n\)
Solution:
1. Step 1: Determine the sum of the mass numbers on the left side of the equation. The sum numbers on the left side of the equation should equal the sum of the mass numbers on the right side of the equation.
2. Step 2: After finding the mass number (A), you may need to solve for the atomic number (Z). This is done by setting the sum of the atomic numbers on the left side equal to the sum of the atomic numbers on the right side. Finding the atomic number enables you to determine which element is missing from the nuclear reaction.
General format for a nuclear particle that is in nuclear reactions:
$$^{A}_{Z}X$$
A = Mass Number (total # of protons and neutrons), Z = Atomic Number (total # of protons), X = Chemical element that corresponds to atomic number
a) \(^{7}_{3}Li+?\rightarrow2^{4}_{2}He\)
Step 1: Sum of the mass numbers of the reactant side: 7+x Sum of the mass numbers on the products side: 2•4= 8
Set the mass numbers of the left and right sides equal to one another: 7+x= 8
x(mass number of unknown nuclear particle)= 8-7 =1
Therefore: \(^{1}_{?}X\)
Step 2: Sum of the atomic numbers on the reactants side: 3+x Sum of the atomic numbers on the products side: 2•2= 4
Set the mass number of the left and right sides equal to one another: 3+x= 4
x(atomic number of unknown nuclear particle)= 4-3 =1
Therefore: \(^{1}_{1}X\)
Since you now know that the atomic number of the nuclear particle is 1, using a periodic table, find which element's atomic number corresponds to this specific atomic number (1). In this case, the element is hydrogen and so the final written form of this nuclear particle is \(^{1}_{1}H\) and the complete nuclear equation is:
$$^{7}_{3}Li+^{1}_{1}H\rightarrow2^{4}_{2}He$$
b) \(^{14}_{6}C\rightarrow^{14}_{7}N+?\)
Step 1: Sum of the mass numbers of the reactant side: 14 Sum of the mass numbers on the products side: 14+x
Set the mass numbers of the left and right sides equal to one another: 14 = 14+x
x(mass number of unknown nuclear particle)= 14-14= 0
Therefore: \(^{0}_{?}X\)
Step 2: Sum of the atomic numbers on the reactants side: 6 Sum of the atomic numbers on the products side: 7+x
Set the mass number of the left and right sides equal to one another: 6 = 7+x
x(atomic number of unknown nuclear particle)= 6-7 = -1
Therefore: \(^{0}_{-1}X\)
This particle's mass number is equal to 0 and its atomic number is -1. From this information, we know that the missing nuclear particle is a β particle,\(^{0}_{-1}e^{-}\), which is an electron that is emitted by an unstable nucleus. The complete nuclear reaction is:
$$^{14}_{6}C\rightarrow^{14}_{7}N+^{0}_{-1}e^{-}$$
c) \(^{27}_{13}Al+^{4}_{2}He\rightarrow?+^{1}_{0}n\)
Step 1: Sum of the mass numbers of the reactant side: 27+4=31 Sum of the mass numbers on the products side: x+1
Set the mass numbers of the left and right sides equal to one another: 31= x+1
x(mass number of unknown nuclear particle)= 31-1= 30
Therefore: \(^{30}_{?}X\)
Step 2: Sum of the atomic numbers on the reactants side: 13+2=15 Sum of the atomic numbers on the products side: x+0= x
Set the mass number of the left and right sides equal to one another: 15 = x
x(atomic number of unknown nuclear particle)=15
Therefore: \(^{30}_{15}X\)
Since you now know that the atomic number of the nuclear particle is 15, using a periodic table, find which element's atomic number corresponds to this specific atomic number (15). In this case, the element is phosphorous and so the final written form of this nuclear particle is \(^{30}_{15}P\) and the complete nuclear equation is:
$$^{27}_{13}Al+^{4}_{2}He\rightarrow^{30}_{15}P+^{1}_{0}n$$
d) \(^{250}_{96}Cm\rightarrow?+^{98}_{38}Sr+4^{1}_{0}n\)
Step 1: Sum of the mass numbers of the reactant side: 250 Sum of the mass numbers on the products side: x+98+4(1)= x+102
Set the mass numbers of the left and right sides equal to one another: 250= x+102
x(mass number of unknown nuclear particle)= 250-102= 148
Therefore: \(^{148}_{?}X\)
Step 2: Sum of the atomic numbers on the reactants side: 96 Sum of the atomic numbers on the products side: x+38
Set the mass number of the left and right sides equal to one another: 96= x+38
x(atomic number of unknown nuclear particle)= 96-38= 58
Therefore: \(^{148}_{58}X\)
Since you now know that the atomic number of the nuclear particle is 58, using a periodic table, find which element's atomic number corresponds to this specific atomic number (58). In this case, the element is cerium and so the final written form of this nuclear particle is \(^{148}_{58}Ce\) and the complete nuclear equation is:
$$^{250}_{96}Cm\rightarrow^{148}_{58}Ce+^{98}_{38}Sr+4^{1}_{0}n$$
Q20.1.1
Question:
Identify the oxidation state of the atoms in the following compounds:
a) PCl3
b) CO32−
c) H2S
d) S8
e) SCl2
f) Na2SO3
g) SO42−
Background Information:
An atom’s oxidation state is representative of the number of electrons that it can lose, gain, or share when it is chemically bonded with another element’s atom, forming a compound. Oxidation state allows us to determine the changes that occur in redox reactions as well as balance redox reactions.
Below are some of the rules to determining an atom’s oxidation number:
-
The oxidation state of an element that is not combined with another element Fe, H2, O2, P4, S8 is zero (0).
-
The oxidation state of oxygen in a compounds is -2, except for when it is in peroxides like H2O2, and Na2O2, in this case the oxidation state for O is -1.
-
The oxidation state of hydrogen is +1 in its compounds, except for metal hydrides, such as NaH and LiH, where the oxidation state for H is -1.
-
The net charge on a molecule of ion is equal to the algebraic sum of the oxidation state of all the atoms present in the species.
-
-
+1 for alkali metals (Group 1): (Li, Na, K, Rb, Cs)
-
+2 for alkaline earth metals (Group 2): (Be, Mg, Ca, Sr, Ba)
-
The oxidation number of fluorine is always –1. Chlorine, bromine, and iodine usually have an oxidation number of –1, unless they are combined with an oxygen or fluorine atom
-
-
The oxidation number of a monatomic ion is equal to the charge on the ion
Solution:
a) PCl3
According to Rule #5 above, chlorine has an oxidation state of -1. There are 3 chlorine atoms present in this compound and so chlorine has a total oxidation state of (-1)3=-3. Because this is a neutral compound, we know that the net charge of the molecule must equal to 0 (Rule #4). Therefore we can find the oxidation state of phosphorous because :
O.S.(P)+O.S(Cl)=net charge
O.S.(P)+(-3)=0
O.S.(P)=+3
Oxidation state of chlorine atom=-1, total oxidation state of Chlorine atoms: -3
Oxidation state of phosphorous atom=+3
b) CO32−
According to Rule #2, oxygen has an oxidation number of -2. Since there are 3 oxygen atoms, the overall oxidation state of the oxygens is (-2)3=-6 Because the overall molecule has a net charge of 2- , carbon must have an oxidation state of (-2)-(-6)=+4.
Oxidation state of oxygen atom=-2
Oxidation state of carbon atom=+4
c) H2S
According to Rule #3, hydrogen has an oxidation state of +1. Since there are two hydrogen atoms, the total oxidation state of the hydrogens is (+1)2=+2. The overall molecule has a net charge of 0, and so the oxidation state of the sulfur atom is 0-(+2)=-2.
Oxidation state of hydrogen atom=+1
Oxidation state of sulfur atom=-2
d) S8
Since this is an elemental form of sulfur, according to Rule #1, the sulfur atom would have an oxidation state of 0.
Oxidation state of sulfur atom=0
e) SCl2
According to Rule #5 above, chlorine has an oxidation state of -1. There are 2 chlorine atoms present in this compound and so chlorine has a total oxidation state of (-1)2=-2. This is a neutral compound, so the net charge of the molecule is equal to 0. Therefore, the oxidation state of the sulfur atom is 0-(-2)-+2.
Oxidation state of chlorine atom=-1
Oxidation state of sulfur atom=+2
f) Na2SO3
Sodium is an alkali metal, and so one Na atom has an oxidation state of +1; the overall oxidation state of the two sodium atoms in this compound is equal to (+1)2=+2. Oxygen atom has an oxidation state of -2, and since there are 3 oxygen atoms, the total oxidation state of the oxygen atoms in this compound is (-2)3=-6. This is a neutral molecule with a net charge of 0, and so the oxidation state of the sulfur atom is 0-(+2)-(-6)=+4.
Oxidation state of sodium atom=+1
Oxidation state of sulfur atom=+4
Oxidation state of oxygen atom=-2
g) SO42−
According to Rule #2, the oxidation state of an oxygen atom is -2. The overall oxidation state of 4 atoms of oxygen is (-2)4=-8. The net charge of the compound is 2-, and so the oxidation state of the sulfur atom is -2-(-8)=+6.
Oxidation state of sulfur atom=+6
Oxidation state of oxygen atom=-2
Q20.4.19
Question:
Carbon is used to reduce iron ore to metallic iron. The overall reaction is as follows:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)+3C(s)\rightarrow4Fe(l)+3CO_{2}(g)+2xH_{2}O(g)$$
Write the two half-reactions for this overall reaction.
Solution:
1. Step 1: Separate the half-reactions, one for oxidation and the other for reduction. Identify which element is oxidized and which element is reduced, then separate the overall equation into the two equations as shown below:
Reduction Half-Reaction:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow4Fe(l)+2xH_{2}O(g)$$
The oxidation number of iron on the left side of the equation is equal to +3 because oxygen is more electronegative than iron, making its oxidation number -2. Since the charge of the overall molecule is neutral (0), the two iron atoms must have an oxidation number of +3 each to balance the charge of the three oxygen atoms (-6). The oxidation number of iron on the right side of the equation is 0 since it is a free element. The iron in this half-reaction is reduced because it gains electrons; the oxidation number of iron decreases from +3 in the reactants side to 0 in the products side.
Oxidation Half-Reaction:
$$3C(s)\rightarrow3CO_{2}(g)$$
The oxidation state of the carbon on the left side of the chemical equation is 0 because it is a free element. The oxidation number of carbon on the right side is +4 since oxygen's oxidation number -2. The charge of the overall molecule is neutral (0), so carbon must be +4 to balance the charge of the two oxygen atoms (-4). The carbon in this half-reaction is oxidized because the carbon atom loses electrons; the oxidation number of carbon increases from 0 in the reactant side to +4 in the products side.
2. Balance all elements aside from oxygen (O) and hydrogen (H).
Reduction Half-Reaction:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow4Fe(l)+2xH_{2}O(g)$$
Oxidation Half-Reaction:
$$3C(s)\rightarrow3CO_{2}(g)$$
3. Add H2O to balance oxygen. In the reduction half-reaction, 6 H2O molecules are added to the products side of the reaction equation to balance the oxygen molecules on the reactants side of the reaction. In the oxidation half-reaction, 6 H2O molecules must be added to the reactants side of the reaction to balance the oxygen molecules on the products side of the reaction.
Reduction Half-Reaction:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow4Fe(l)+2xH_{2}O(g)+6H_{2}O(l)$$
Oxidation Half-Reaction:
$$3C(s)+6H_{2}O(l)\rightarrow3CO_{2}(g)$$
4. Balance hydrogen by adding hydrogen protons (H+). For the reduction half reaction, 12 hydrogen protons are added to the left side of the iron reaction to balance the 16 hydrogens on the right side. For the oxidation half reaction, 12 protons need to be added to the right side of the carbon reaction to balance the 12 hydrogens on the left side.
Reduction Half-Reaction:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)+12H^{+}(aq)\rightarrow4Fe(l)+2xH_{2}O(g)+6H_{2}O(l)$$
Oxidation Half-Reaction:
$$3C(s)+6H_{2}O(l)\rightarrow3CO_{2}(g)+12H^{+}(aq)$$
5. Balance the charge of each equation using electrons. The reduction half reaction has a +12 charge on the left side of the reaction due to the 12 protons, while the other side has a neutral (0) charge, so 12 electrons must be added to the left side of the reaction to balance the charges on both sides so that the overall charge of the reaction equals 0. The oxidation half reaction has a +12 on the right side of the reaction due to the 12 protons, while the other side has a neutral (0) charge, so 12 electrons must be added to the right side of the reaction to balance the charges on both sides of the equation so that the overall charge of the reaction equals to 0.
After completing this step, you have found the balanced half reactions of the overall reaction.
Reduction Half-Reaction:
$$2Fe_{2}O_{3}\cdot xH_{2}O(s)+12H^{+}(aq)+12e^{-}\rightarrow4Fe(l)+2xH_{2}O(g)+6H_{2}O(l)$$
Oxidation Half-Reaction:
$$3C(s)+6H_{2}O(l)\rightarrow3CO_{2}(g)+12H^{+}(aq)+12e^{-}$$
Q20.9.4
Question:
Two solutions, one containing Fe(NO3)2·6H2O and the other containing the same molar concentration of Fe(NO3)3·6H2O, were electrolyzed under identical conditions. Which solution produced the most metal? Justify your answer.
Solution:
Electrolysis is the process where an electric current is passed through an ion-containing solution, creating a redox reaction in which one substance loses electron(s) and the other substance gains electron(s). This process is carried out in an electrolytic cell, which is an apparatus that consists of negative and positive electrodes each dipped into a solution that contains both positive and negative charged ions. During electrolysis, an external voltage is applied to the apparatus, to drive a non-spontaneous reaction. In the example given, this non-spontaneous reaction is where iron cations are reduced to form iron metal. Electrons will flow from the anode (oxidation occurs here) to the cathode (reduction occurs here) where the iron cations will gain these electrons and be deposited on the electrode, forming iron metal.
To identify which solution produced the most metal, we must first write out the reduction half reactions of each iron compound.
Fe(NO3)2·6H2O: \(Fe^{2+}(aq)+2e^{-} \rightarrow Fe(s)\)
Fe(NO3)3·6H2O: \(Fe^{3+}(aq)+3e^{-} \rightarrow Fe(s)\)
Because we are told that both of these iron compounds are electrolyzed under identical conditions, we know that the same amount of electrons were transferred in both systems. Let \(x\)=the total moles of electrons transferred, and using stoichiometric ratio, then:
in the electrolysis of Fe(NO3)2·6H2O, \(\frac{x}{2}\) moles of Fe metal are produced
in the electrolysis of Fe(NO3)3·6H2O, \(\frac{x}{3}\) moles of Fe metal are produced
Therefore, the electrolysis of Fe(NO3)2·6H2O will produce the most metal.
Q20.8.3
Question:
Why is it important for automobile manufacturers to apply paint to the metal surface of a car? Why is this process particularly important for vehicles in northern climates, where salt is used on icy roads?
Solution:
The paint that is applied to the metal surface of a car is important because it prevents corrosion by preventing oxygen and water from coming into direct contact with the car's metal surface. Corrosion is a naturally occurring process in which metals are oxidized in the presence of moisture or air. In northern climates, the salt used on the icy roads lowers the freezing point of water, and so the roads won't be as icy and skidding is less likely to happen. Because of this, paint is even more necessary in these climates because salt is an electrolyte that increases water's conductivity, facilitating the flow of electric current between cathodic and anodic sites. So without the paint, the rate of corrosion of the metal on the car's surface would increase. The paint therefore forms a protective layer on top of the metal, keeping the oxygen and water from directly touching the metal and causing its corrosion.

