Extra Credit 20
- Page ID
- 82931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.9
- An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
- An active (metal) electrode was found to lose mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
Q17.2.9
Solution:
First, let's define anode and cathode. (Phase II: added introduction) In Electrochemical cells, the anode is the place where the oxidation half-reaction occurs, and the cathode is the place where the the reduction half-reaction happens. (Phase II reworded a little bit + added "half" to a reaction). Since oxidation reactions are defined by an increase in oxidation state (a loss of electrons) (Phase II: deleted that an increase in oxidation state is accompanied with a loss of electrons: this is a little wrong because increase in oxidation state is a result of losing electrons), electrons flow from the anode to the cathode. Reduction reactions are defined by a decrease in oxidation state and are accompanied with a gain of electrons, meaning that electrons flow into the cathode.

In this system (Phase II: increased the picture to allow greater visibility) above, the Cu acts as the electrode in the anode. When the oxidation (Phase II: ''redox" changed to "oxidation") reaction proceeds, Cu is electrolyzed into a cation (positively charged ion (added by Phase II), Cu2+, and two electrons (Phase II: deleted a typo). As a result, the electrode in the anion loses mass because some of the solid Cu, which was part of an electrode is converted into aqueous Cu2+(Phase II: additional explanation about states of Cu is added). In the cathode, Ag acts as the electrode with the aqueous form of Ag+ in the surrounding solution. When the electrons flow into the cathode, Ag+ (aq) combines with the electrons to form Ag(s). The newly formed Ag(s) now bind and plate to the Ag electrode and add to its mass.
Now, to the question: If an electrode is found to gain mass as a redox reaction proceeds, we can infer that the electrode must have been (Phase II: deleted some unnecessary words) the cathode because the electrons flow into the cathode and when this happens the Ag+(aq) forms Ag(s) and plates onto the electrode, gaining mass. The electrode was part of the cathode.
If an electrode is found to lose mass as a redox reaction proceeds, we can infer that the electrode must have been the anode because the electrode in the anode supplies electrons and when it supplies electrons, it forms an ion, which is dissolved into the aqueous solution, losing mass. The electrode was part of the anode.
Note that this answer is applicable to any functional electrochemical cell. For instance, if we used a Zinc Copper cell, it would function in a very similar way, except that Zn acts as the anode and the Cu acts as the cathode.
The general form of this answer is oxidation occurs in the anode. Oxidation results in a loss of electrons and therefore, a loss of mass (Phase II: information about mass added). When the electrode donates its electrons, it forms a cation. When the metal forms a cation, it removes atoms from the electrode, reducing its mass.
Reduction occurs in cathode. Reduction results in a gain of electrons. When the cations in the aqueous solution combine with the electrons flowing from the anode it forms a metal solid. This metal solid binds to the electrode and increases its mass.
[Phase II: mostly fixed wording, added more clarification information]
Q19.1.18
Describe the electrolytic process for refining copper.
Q19.1.18
Solution:
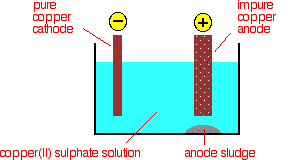
Copper ore can be refined through the process of electrolysis. The impure copper, often in the form of copper ore, acts as the anode in an electrolyte bath of copper sulfate and sulfuric acid. The cathode is a sheet of pure copper.
When a current is applied to the system, the copper cations, \(\text{Cu}^+_{(aq)}\) , from the copper sulfate \(\text{CuSO}_{4}\) (Phase II: changed the format) in the electrolytic solution are attracted to the cathode. As they gain the electrons from the current applied to the system, they form solid copper and deposit themselves as pure copper atoms on the cathode.
At the anode, the copper atoms donate their electrons to the solution, and the solid copper dissolves into the solution as copper ions, thereby replacing the copper ion concentration in the solution.
Q19.3.10
Would you expect the complex [Co(en)3]Cl3 to have any unpaired electrons? Any isomers?
S19.3.10
Solution:
- The first step in determining if the metal complex has any unpaired electrons is to break it up into its cations and anions. When we do this, we get \(\text{[Co}{(en)}_{3}\text{]}^{3+}\) and \( \mathrm{3Cl^{-}}\).
- Now that we've broken up the complex, we want to determine what the oxidation state of Co is. To do this, we must know that (en) has a charge of 0. Because of that and the fact that if that compound paired with \( \mathrm{3Cl^{-}}\) produce neutral complex (Phase II: added needed information about determining the charge) we know that Cobalt must of have an oxidation state of 3+ or \( \mathrm{Co^{3+}}\) .
- Next, we want to determine the electron configuration (Phase II: deleted unneeded words). Cobalt is located in the 3d block and has the electron configuration of \(\text{[Ar]4s}^{2}\text{3d}^{7}\) (Phase II: changed the electro configuration to noble gas configuration, since it's the best representative of the electrons that matter the most when determining unpaired electrons).
- However since it has a 3+ charge we must remove 3 electrons. When removing the electrons, remove the electrons with the highest energy state, which means that we must remove 2 electrons from the s-orbital and one from the d-orbital. We end up with: (Phase II: deleted unnecessary step, and gave more concise form + added some transitions)
- \( \mathrm{Co^{3+}} = \text{3d}^{6}\)
- Now that we have the electron configuration, we know that there are 6 electrons in the d-orbital.
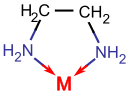
- Next we need to determine the geometry of cobalt. It is bound to 3 ethylenediamine (en), a bidentate ligand - this type of ligand binds forms two bonds with a metal ion (Phase II: added the explanation of what bidentate means). These ligands have two donor atoms which allow them to bind to the central metal atom at two points meaning that it has two bonds with the cobalt (Phase II: corrected typo). Since there are 3 bidentate ligands, cobalt must have a coordination number of six and, therefore, must be octahedral in its geometry. The figure below shows how en binds to a metal. (Phase II: added explanation for the picture).
- The next step is to determine if the ligands in the metal complex are strong or weak field ligands by referring (Phase II; typo corrected) to the spectrochemical series. From the list, we know that en is a strong field ligand and, therefore, the Δo is large. When Δo is large (strong field ligand), the electrons will first fill the lower energy d orbitals before any electrons are placed on the higher energy d orbitals.
- Next we determine if there are any unpaired (Phase II: typo corrected) electrons by using the crystal field splitting for octahedral complexes. We fill the lower energy d orbitals first with six electrons and get (Phase II: added that there is 6 electrons).

- And in this diagram, we see that there are no unpairred electrons.
- Before we can determine if there are any isomers, we must understand what isomers are and what types there are. An isomer is a molecule that has the same molecular formula but a different geometric structure. There are two groups of isomers: structural and stereoisomers.
- Structural isomers have different bonds between the central metal and the ligands, resulting in a different geometry (Phase II: format modified). Ionization isomers occur when the ligands bonded to the central metal switch places with the ions outside of the coordination sphere. Coordination isomers occur when there is a change in ligands between cation and anion coordinate sphere in a multi-coordinate complex chemicals. Linkage isomers occur with ambidentate ligands that are capable of forming bonds in more than one way. The ligand that is attached to the central metal can bind in different ways (Phase II: deleted some information about bonding of atom because it seemed inaccurate).
- Stereoisomers have the same bonds but still result in a different orientation because of a different arrangement of their ligands. Of these there are geometric and optical isomers. Geometric isomers differ in which ligands are next to each other or across from each other; they differ from their the bond angle between identical ligands.Examples of these isomers are cis and trans and mer and fac. Optical isomers are compounds that have the same structure but has different mirror images. They are capable of rotating linear polarized light.
- Now, back to the problem. (Phase II: added this transition) So the first step is to determine if the complex (Phase II: deleted "it", and chose a more specific term) is a structural isomer. It can form an ionization isomer because the \( \mathrm{Cl^{-}}\) can swap places to form \(\text{[Co}{(en)}_{2}\text{Cl}_{2}\text{]Cl(en)}\) (Phase II: changed the subscripts). The complex cannot form a coordination isomer because it doesn't have an anion complex. It cannot be a linkage isomer because there the ligands aren't ambidentate, and there is only one way for (en) and \( \mathrm{3Cl^{-}}\) to bind to Co.
- Now we want to determine if there are any stereoisomers. We know that it isn't a geometric isomer because there is no other way to arrange the ligands as cis and trans or mer and fac. The coordinate complex has an stereoisomer, because it has no plane of symmetry.
- Next we can determine if there are any optical isomers by creating a mirror image of the molecule and trying to superimpose the new molecule over the original. We can also determine if there are any optical isomers by determining if there is a mirror plane within the molecule. If the molecule is not superimposable or does not have a mirror plane, then the molecule is chiral (Phase II: changed "achiral" to the right term – achiral means that the complexes are superimposable) or has an (Phase II: typo corrected) optical isomer.
- By following the previously (Phase II: typo corrected) stated steps, we cannot find a mirror plane or superimpose the mirror molecule over the original (so it would look the same as the original – added by Phase II) so the molecule is chiral (Phase II: changed "achiral" to the right term – achiral means that the complexes are superimposable) or is an optical isomer.
[Phase II: fixed a lot of punctuation]
Q12.4.10
The reaction of compound A to give compounds C and D was found to be second-order in A. The rate constant for the reaction was determined to be 2.42 L/mol/s. If the initial concentration is 0.500 mol/L, what is the value of t1/2?
Q12.4.10
Solution:
A →C +D
The equation for second-order (Phase II: specified that this equation is for the 2nd order) half life is given by \[t_{1/2} = \frac{1}{[Ao]k} \]
Since we know that the rate constant k, is 2.42 L/mol/s. We also know that the initial concentration is 0.500 mol/L. (Phase II - typo fixed) We can simply plug in our values to find half life.
\[t_{1/2} = \frac{1}{(0.500mol/L)(2.42L/mol/s)} \]
\[t_{1/2} = .8264s \]
t1/2 = 0.826s (corrected the sig figs - added by Phase II) (also check if the units make sense –they do)
Q21.2.5
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
- 25 protons, 20 neutrons, 24 electrons
- 45 protons, 24 neutrons, 43 electrons
- 53 protons, 89 neutrons, 54 electrons
- 97 protons, 146 neutrons, 97 electrons
Q21.2.5
Solution:
Each element can be represented by the notation , where A is the mass number, Z is the atomic (or proton – added by Phase II) number, and X is the element. Although we aren't explicitly given the mass number, we can calculate this by finding the sum of the number of protons and neurons. We can find the charge of the atom by finding the difference of the number of protons and electrons.
1. 25 protons, 20 neutrons, 24 electrons
- First find A:
- A= number of protons + number of neutrons
- A= 25+20= 45
- Find Z:
- Z= number of protons
- Z=25
- Find the charge:
- Charge= number of protons – numbers of electrons
- Charge=25-24=+1
- With this information we know that this atom must be Mn because only Mn has 25 protons. So X=Mn. Combine this information in the notation stated above to get:
\[{45 \atop 25} Mn{+ \atop}\]
2. 45 protons, 24 neutrons, 43 electrons
Use same procedure as in 1 (added by Phase II)
- First find A:
- A= number of protons + number of neutrons
- A= 45+24= 69
- Find Z:
- Z= number of protons
- Z=45
- Find the charge:
- Charge= number of protons- numbers of electrons
- Charge=45-43=+2
- Charge= number of protons- numbers of electrons
- With this information we know that this atom must be Rh because only Rh has 45 protons. So X=Rh. Combine this information in the notation stated above to get:
\[{69 \atop 25} Rh{2+ \atop}\]
3. 53 protons, 89 neutrons, 54 electrons
Use same procedure as in 1 (added by Phase II)
- First find A:
- A= number of protons + number of neutrons
- A= 53+89= 142
- Find Z:
- Z= number of protons
- Z=53
- Find the charge:
- Charge= number of protons- numbers of electrons
- Charge=53-54=-1
- With this information we know that this atom must be Rh because only Rh has 45 protons. So X=Rh. Combine this information in the notation stated above to get:
\[{142 \atop 53} I{- \atop}\]
4. 97 protons, 146 neutrons, 97 electrons
Use same procedure as in 1 (added by Phase II)
- First find A:
- A= number of protons + number of neutrons
- A= 97+146= 243
- Find Z:
- Z= number of protons
- Z=97
- Find the charge:
- Charge= number of protons- numbers of electrons
- Charge=97-97=0
- With this information we know that this atom must be Rh because only Rh has 45 protons. So X=Rh. Combine this information in the notation stated above to get:
\[{243 \atop 97} Bk\]
Q21.5.8
The mass of a hydrogen atom \[ ^{1}_{1}\text{H} \] is 1.007825 amu; that of a tritium atom \[ ^{3}_{1}\text{H} \] is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of \[ ^{4}_{2}\text{He} \] produced is released by the following fusion reaction:
\[ ^{1}_{1}\text{H} + ^{3}_{1}\text{H} \rightarrow ^{4}_{2}\text{He}\]
Q21.5.8
Solution:
- The first step of this problem is to recognize that this is a nuclear reaction problem and will require the equation \(\text{E=mc}^2\) which can be rewritten as \(\text{ΔE=Δmc}^2\) (where, E is energy, c is speed of light and Δm is change in mass – Phase II added the explanation of the formula)
- The next step is to find Δm. This is equal to the mass of free nucleons – mass of nucleus. (Phase II: deleted some unneeded material)
- Δm=(1.007825 + 3.01605) amu - 4.00150 amu
- Δm=0.022375 amu (Phase II: corrected mistake in units)
- Δm=0.022375 g/mol (amu is equal to g/mol – clarification added by Phase II)
- C is equal to 2.9979 x108 m/s (Phase II: some content modified)
- Now, lets combine this information to get ΔE
- ΔE=(0.022375 g/ mol)*(2.9979 x108 m/s)2
- ΔE=2.01093 x1015 J/mol
- ΔE=2.01093 x1012 kJ/mol
- check the sig figs and units to make sure everything looks right (–added by Phase II)
We can see that even if the change in mass might seem insignificant, a lot of energy is released in a nuclear reaction – conclusion added by Phase II.
Q20.4.6
Explain why E° values are independent of the stoichiometric coefficients in the corresponding half-reaction.
Q20.4.6
Solution:
First of all, we need to understand what kind of properties exist and what they mean in terms of influences – (sentence added by Phase II). Properties can be categorized as extensive or intensive (Phase II - typo fixed) properties: An extensive property is one that varies as the amount of material changes such as mass or volume. An intensive property is one that doesn't change as the amount of material change such as temperature or pressure. E°, or standard cell potential, is a measure of the driving force of the redox reaction. Standard cell potential can be categorized (Phase II - typo fixed) as an intensive property so because of that property, it is independent of the amount of material and therefore independent of the stoichiometric coefficients, which represents amount of reactants and products (Phase II: added the explanation for stoichiometry).
Q20.7.4
Why are galvanic cells used as batteries and fuel cells? What is the difference between a battery and a fuel cell? What is the advantage to using highly concentrated or solid reactants in a battery?
Q20.7.4
Solutions:
Since galvanic cells can be self-contained and portable, they can be used as batteries and fuel cells. A battery, storage cell, is a galvanic cell or series of galvanic cells connected to one another that contain the reactants needed to produce electricity. A fuel cell is a galvanic cell that requires the constant external supply of reactants to generate electricity.
The advantage of having highly concentrated or solid reactants is that the concentrations of the reactants and the products don't change greatly as (Phase II: typo corrected) the batter is discharged. As a result of this, the output voltage remains fairly constant while being used.

