Extra Credit 13
- Page ID
- 82923
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.2
Given the following cell notations, determine the species oxidized, species reduced, and the oxidizing agent and reducing agent, without writing the balanced reactions.
a. Mg(s)|Mg2+(aq)||Cu2+(aq)|Cu(s)
b. Ni(s)|Ni2+(aq)||Ag+(aq)|Ag(s)
S17.2.2
In a Galvanic cell the species that acts as the oxidizing agent is on the left of the double lines ( which represents a salt bridge between the two half reactions) and the species being reduced sits to the right of the double lines. The single vertical lines on each side represents the phase changes of the species. The oxidizing agent accepts electrons making the species become more reduced. The reducing agent gives off electrons which makes it the species oxidized.
a)
Mg(s) is oxidized because it loses electrons and becomes more positively charged. In the reaction it changes from Mg to Mg2+, showing electron loss. The electrons are added on the right of the cell equation.
Mg(s)→Mg2+(aq)+2e-
Cu2+ (aq) is reduced because it gains electrons and becomes more negatively charged. in the reaction it changes from Cu2+ to Cu. The electrons are added to the left in the cell equation.
Cu2+(aq)+2e-→Cu(s)
Mg(s) is the reducing agent as it gives away its electrons to be oxidized, allowing Cu2+ to gain those lost electrons and be reduced.
Cu2+ (aq) is the oxidizing agent as it takes away Mg's electrons, in order to be reduced, thereby causing Mg to be oxidized.
b)
Ni(s) is oxidized because it loses electrons and becomes more positively charged. in the reaction it changes to Ni2+. It's to the left.
Ni(s)→Ni2+(aq)+2e-
Ag+(aq) is reduced because it gains electrons and becomes more negatively charged. in the reaction it changes to Ag. It's to the right.
Ag+(aq)+e-→Ag(s)
Ni(s) is the reducing agent as it gives away its electrons to be oxidized, allowing Ag+ to gain those lost electrons and be reduced.
Ag+(aq) is the oxidizing agent as it takes away Ni's electrons, in order to be reduced, thereby causing Ni to be oxidized.
An extra way to tell is that the Anode half reaction of the cell of the cell is normally written on the right and the anode reaction is the one that is oxidized.
The Cathode half reaction of the cell is normally written on the left and is the one that gets reduced. Also whatever is oxidized will almost always be the reducing agent and vise verca.
Q19.1.11
Iron(II) can be oxidized to iron(III) by dichromate ion, which is reduced to chromium(III) in acid solution. A 2.5000-g sample of iron ore is dissolved and the iron converted into iron(II). Exactly 19.17 mL of 0.0100 M Na2Cr2O7 is required in the titration. What percentage of the ore sample was iron?
S19.1.11
First we have to figure out our oxidation and reduction half reactions:
Fe2+ → Fe3+
Fe becomes more positively charged so it's the oxidation half, but it's not fully balanced in terms of charge so we need to add an electron to the right side.
Fe2+→Fe3++e-
Now both sides charges are equal.
Now we figure out the reduction half reaction:
Cr2O72-→2Cr3+
At this point this equation is not fully balanced out in terms of oxygens on both sides, so we need to add water to the right side.
Cr2O72-→2Cr3++7H2O
But now we need to balance the H atoms, so we need to add some H+ to the left.
Cr2O72-+14H+→2Cr3++7H2O
Now we need to balance out the charges like we did before with Fe by adding 6e to the left
Cr2O72-+14H++6e-→2Cr3++7H2O
Now we want to add the 2 equations but first we need to ensure the electrons we added will fully cancel out, so we need to multiply the oxidation half reaction by 6
6Fe2+→6Fe3++6e-
Now we can add the equations to get
6Fe2++Cr2O72-+14H++6e-→6Fe3++6e-+2Cr3++7H2O
Now we can cancel out both the 6e from both sides.
6Fe2++Cr2O72-+14H+→6Fe3++2Cr3++7H2O
This is our final equation.
Then we find the moles of the Na2Cr2O7 used by multiplying .01917L ( this is 19.17ml divided by 1000 to change it from ml to L) by .01M.
mole Na2Cr2O7 which means 1.917x10-4= moles Cr2O72-
Then we multiply that by 6 because there are 6 moles of iron per every mole of chromate.
= .0011502 mole Fe
Then we find what the actual mole of iron ore we have are by dividing the grams of iron by its atomic weight.
= .0447667652 mole Fe
Then we divide the moles of iron we calculated from the chromium titrated by the moles of iron calculated by the actual sample of iron we were given. the equation is (observed)/(actual).
= .02569
Then, to make it a percent, we multiply by 100.
= 2.57%
Q19.3.3
Give the oxidation state of the metal, number of d electrons, and the number of unpaired electrons predicted for [Co(NH3)6]Cl3.
Answer:
Oxidation state: NH3 is neutral meaning it has no charge so it adds no charge to the complex. Cl has a charge of -1 and we have 3 Cl, so in total it adds a -3 charge to the complex. The complex as a whole is neutral and has no charge, so we have to make sure that all the parts' charges add up to 0. Currently we have a -3 charge, so we have to cancel that out with the Co atoms having a charge of +3. This means Co has an oxidation state of +3.
# of d electrons: We look at the metal in the complex when trying to count the d electrons, so for this complex we're focusing on Co. Co normally has 27 electrons as you can find on the periodic table, but, as we established earlier, in this complex Co has an oxidation number of +3 so that means it has 3 less electrons to cancel out the positive charge from the protons in the atom. So Co now has only 24 electrons. 1s2 2s2 2p6 3s2 3p64s2 3d10 this is the sequence for how electrons are organized in the orbitals, the exponents represent the number of electrons that can be held in each suborbital. So we place our 24 electrons into each suborbital so that each one has the full number of electrons it can hold( based on the exponent e.g. 1s2can hold 2 electrons) and we end up with 4 electrons in the 3d orbital. Since the 3d10 orbital is not fully full (it only has 4 electrons and can hold 10) it steals the 2 electrons in the 4s2 orbital meaning it has 4+2=6 electrons left in the d orbital. So our final answer is that there are 6 electrons in the d orbital.
# of unpaired electrons: First of all this complex is octahedral because it has a central atom with 6 NH3 ligands bonded to it. Secondly NH3 is a strong field ligand meaning the complex will be a low spin. the electrons will prefer to pair together across all the empty orbitals before they fill an orbital on their own, meaning there will be much fewer unpaired electrons than if the complex had a weak field/high spin ligand. There are 5 planes the electrons can split into, so with this we split our 6 d electrons up so that 2 electrons sits on each of the 5 planes. this means we have 0 left unpaired since the 6 will pair up to only take up 3 of the 5 planes. So our final answer is that there are 0 unpaired d electrons.
.jpg?revision=1&size=bestfit&width=180&height=230)
Q12.4.3
Use the data provided in a graphical method to determine the order and rate constant of the following reaction:
2P→Q+W
| Time(s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
| [P] (M) | 1.077x10-3 | 1.068x10-3 | 1.055x10-3 | 1.046x10-3 | 1.039x10-3 |
Answer:
We are given concentration over time and we're looking for the order of the reaction and what k is.
To do this we first should figure out the order of the reaction.
First lets graph [p](m) Vs. Time(s) to check if it's a zero order.
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| [P] (M) | 1.077 × 10−3 | 1.068 × 10−3 | 1.055 × 10−3 | 1.046 × 10−3 | 1.039 × 10−3 |
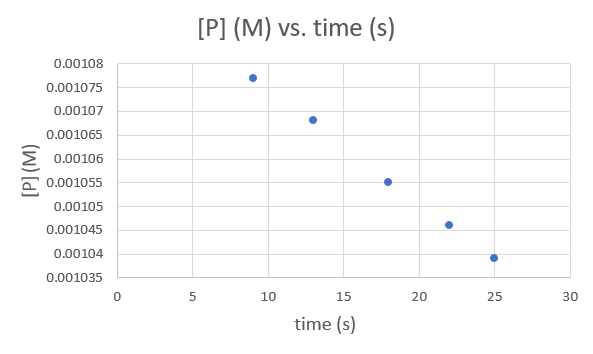.png?revision=1&size=bestfit&width=593&height=354)
This graph is linear which would suggest that this reaction is a zero order but we had better check that it's not linear for any of the other orders.
Next we can try ln[p](m) Vs. Time(s) which will check if it's a first order reaction.
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| ln [P] (M) | -6.83358 | -6.84197 | -6.85421 | -6.86278 | -6.8695 |
.png?revision=1&size=bestfit&width=592&height=354)
So this graph is also straight so it also makes the reaction appear to be a fist order, but the reaction can't be both a zero and first order reaction. This means that we probably won't be able to discover the order of this reaction using graphs, but we should check if it's straight for the second order graph as well before we pass our final judgment.
So finally we'll try 1/[P](m) Vs. Time(s)
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| 1/[P] (M) | 928.5051 | 936.3296 | 947.8673 | 956.0229 | 962.4639 |
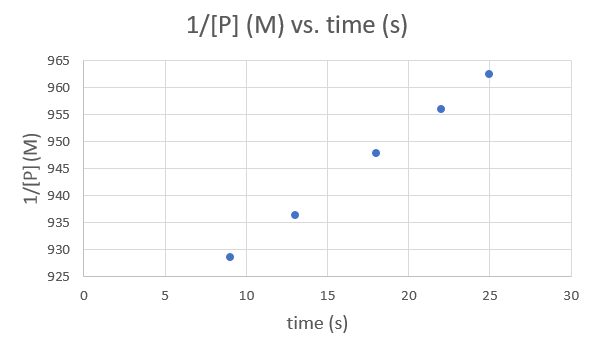.png?revision=1&size=bestfit&width=593&height=353)
So this graph is also linear. This means we can not determine the order of the reaction from graphing the data given. The rate constant k for a zero and first order reaction is the negative of the slope and for a second order reaction it is exactly equal to the slope. This means that since our graphs are inconclusive, we also can not figure out what the rate constant is for this reaction.
Q21.5.1
Write the balanced nuclear equation for the production of the following transuranium elements:
- berkelium-244, made by the reaction of Am-241 and He-4
- fermium-254, made by the reaction of Pu-239 with a large number of neutrons
- lawrencium-257, made by the reaction of Cf-250 and B-11
- dubnium-260, made by the reaction of Cf-249 and N-15
S21.5.1
a. 24195Am+42He→24497Bk+10n
b.23994Pu+1510n→254100Fm+60-1e
c.25098Cf+115B→257103Lr+410n
d.24998Cf+157N→260105Db+410n
Explanation:
a.) First we should write out what we know
24195Am+42He→24497Bk
mass number (top one):
reactants: 241+4=245
products: 244
we need a mass number of 1 to be added to products to equal the reactants
atomic number (bottom one):
reactants: 95+2=97
products: 97
the products and reactants are already equal so we don't need to add anything to either side
Finally we need to add to the product side a particle (42alpha, 0-1beta, 00gamma, 10neutron, 11proton, 0+1positron) that fits the situation. for this one it's a neutron.
24195Am+42He→24497Bk+10n
b.) First we should write out what we know
23994Pu+?10n→254100Fm
mass number (top one):
reactants: 239+?=products: 254
?=254-239=15
therefore we need 15 neutrons added to the reactants and no mass added to the products
23994Pu+1510n→254100Fm
atomic number (bottom one):
reactants: 94
products: 100
so we need to add a -6 to the products to equal the reactants. So we need to use 6 beta particles
23994Pu+1510n→254100Fm+60-1e
c.)First we should write out what we know
25098Cf+115B→257103Lr
mass number (top one):
reactants: 250+11=261
products: 257
so we need to add 4 to the products side
atomic number (bottom one):
reactants: 98+5=103
products: 103
so they are already equal and we don't need to add anything here. Therefore we need to add 4 neutrons to the product side
25098Cf+115B→257103Lr+410n
d.)First we should write out what we know
24998Cf+157N→260105Db
mass number (top one):
reactants: 249+15=264
products:260
so we need to add 4 to the products side
atomic number (bottom one):
reactants: 98+7=105
products: 105
so the products and reactants are equal, so we need to add 4 neutrons to the products side
24998Cf+157N→260105Db+410n
Q12.7.6
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
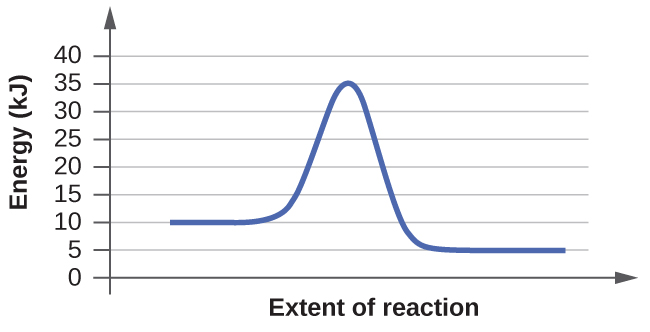
(a)
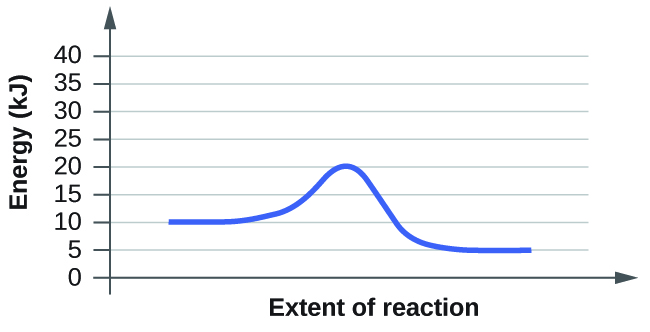
(b)
S12.7.6
- (a) The activation energy starts at 10kj and ends at 35kj. So from an estimation the activation energy is about 25kj total.
- (b) The activation energy starts at 10kj and ends at 20kj. So from this estimation the total activation energy is about 10kj.
Explanation: The delta H energy spans from the plateau at the beginning of the graph that represents the reactants and the plateau at the end of the graph representing the products. For example in both of these graphs the delta H energy goes from 10kj to 5kj. The activation energy, however, is the spike in energy that goes beyond the what the delta H would be. It spans from the top of the delta H to the peak of the curve in the graph. So for these graphs the activation energy is from 10kj to the top energy of the peak.
Q20.3.15
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- 2Cl−(aq) + Br2(l) → Cl2(g) + 2Br−(aq)
- 2NO2(g) + 2OH−(aq) → NO2−(aq) + NO3−(aq) + H2O(l)
- 2H2O(l) + 2Cl−(aq) → H2(g) + Cl2(g) + 2OH−(aq)
- C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Answers:
a.) Half reactions:
initially we have 2Cl--(aq)→Cl2(g) and Br2(l)→2Br--(aq) these two equations need electrons added so the charges on each side are equal.
This ends up giving us our final half reactions of:
oxidation: 2Cl-(aq)→Cl2(g)+2e-
reduction: Br2(l)+2e-→2Br-(aq)
Spontaneous?: To find whether it's spontaneous or not we must use this equation and the standard reduction potentials for these reactions found from a standard reduction potential table:

Galvanic cell: In a galvanic cell diagram the reaction in the anode is written on the right and the reaction in the cathode is written on the left. The left half cell containing the cathode and the reduction half reaction is separated from the right half cell containing the anode and the oxidation half reaction by two vertical lines that represent a salt bridge which allows the charge to flow from one half cell to another so the cell can continue to flow energy. within the two half reaction different phases of species are separated by a single vertical line. each half cell has an electrode to allow the electrons to flow through to power the cell, usually this is made by two different conducting metals that are a part of the half reactions, but sometimes there is a need for an inert electrode (Pt(s) or C(s)) which will be written on the end of the half cell that needs it separated by another single vertical line. Here's the galvanic cell for this reaction:
Pt(s) | Br2(l) | Br-(aq) || Cl-(aq) | Cl2(g) | Pt(s)
b.) Half reactions:
Our initial oxidation and reduction reactions are:
Oxidation: N2O4(g) → NO3-(aq)
reduction:N2O4(g)→ HNO2(aq)
Oxidation: Now we have to balance the equations by adding things like H2O and H+ and electrons:
N2O4 → 2NO3-(aq) This balances the N's
Now we add H2O and H+ to the opposite side in order to balance the O's
N2O4(g)+2H2O(l) → 2NO3-(aq) + 4H+(aq)
Now we need to balance the charges by adding electrons:
N2O4(g)+2H2O(l) → 2NO3-(aq) + 4H+(aq)+2e-
Reduction: first balance N
N2O4(g)→ 2HNO2(aq)
Then balance O and H with H2O and H+
N2O4(g)+2H+(aq)→ 2HNO2(aq)
Finally balance charges with electrons:
N2O4(g)+2H+(aq)+2e-→ 2HNO2(aq)
Spontaneous?: again we use this equation and plug in the standard reduction potentials for these equations. For the anode reaction we have to flip the sign as given on the tables are the oxidation potential for that one. so we get:
anode: N2O4(g)+2H2O(l) → 2NO3-(aq) + 4H+(aq)+2e- Eo= -.79 flipped to be +.79 V
cathode: N2O4(g)+2H+(aq)+2e-→ 2HNO2(aq) Eo = 1.07 V
with this in mind:
Eocell = 1.07 - .79 = .28 V
This is positive meaning it is spontaneous. neither of these are conductive metals so we do need inert electrodes added to each side and we still need a asalt bridge. this looks like this:
Galvanic cell: Pt(s)|N2O4(g)|NO3-(aq)||HNO2(aq)|N2O4(g)|Pt(s)
c.) Half ractions:
Oxidation: 2Cl-(aq)→Cl2(g)
Reduction: 2H2O(l)→H2(g)
Oxidation: we need to add the electrons to equal the charge:
2Cl-(aq)→Cl2(g)+2e-
Reduction: Add H2O and H+ to balance O's
2H2O(l)+2H+(aq)→H2(g)+2H2O(l)
The overall reaction contains OH- molecules meaning we need to add OH- to cancel out the H+ molecules to keep it in a basic solution. Add the same number of OH molecules to both sides.
2H2O(l)+2H+(aq)+2OH-(aq)→H2(g)+2H2O(l)+2OH-(aq)
the OH and H add together to form water, so we now get
2H2O(l)+2H2O(l)→H2(g)+2H2O(l)+2OH-(aq)
Then we cancel out the like terms on both sides:
2H2O(l)→H2(g)+2OH-(aq)
Finally we even out the charge with electrons:
2H2O(l)+2e-→H2(g)+2OH-(aq)
Spontaneous?:
Now we say which is at the anode and which is at the cathode and what their standard reduction potentials are
anode: 2Cl-(aq)→Cl2(g)+2e- Eo = 1.36 V
cathode: 2H2O(l)+2e-→H2(g)+2OH-(aq) Eo = -0.83 V
Now use the Eocell equation:
Eocell = Eocathode -- Eoanode
Eocell= -0.83--1.36= -2.19 V
Eocell is negative so this reaction is not spontaneous in the forward direction, but it is in the reverse. It needs inert electrodes as neither half cell has a conductive metal in its equation. It needs a salt bridge between the two halves. It will look like this:
Galvanic cell: Pt(s) | H2(g) | OH-(aq) || Cl-(aq) | Cl2(g) | Pt(s)
d.) Note: This is a combustion reaction which means it has to be spontaneous
Half reactions:
Oxidation: C3H8(g)→3CO2(g)
Reduction: 5O2(g)→4H2O(g)
Oxidation: we need to balance the O atoms by adding H2O and the necessary H+ atoms:
C3H8(g)+6H2O(l)→3CO2(g)+20H+(aq)
Now we need to balance the charges with electrons:
C3H8(g)+6H2O(l)→3CO2(g)+20H+(aq)+20e-
Reduction: we need to balance the O's by adding H2O and H+
5O2(g)+20H+(aq)→4H2O(g)+6H2O
5O2(g)+20H+(aq)→10H2O(g)
Now we balance the charges with electrons:
5O2(g)+20H+(aq)+20e-→10H2O(g)
Then you should simplify the equation down to
O2(g)+4H+(aq)+4e-→2H2O(g)
Spontaneous?: Now we find the standard reduction potentials and prove that it's spontaneous
anode: C3H8(g)+6H2O(l)→3CO2(g)+20H+(aq)+20e- Eo = ?
cathode: O2(g)+4H+(aq)+4e-→2H2O(g) Eo = 1.229 V
There is no standard reduction potential for the anode equation. The way to calculate it is to find in another table the Gf for the reactants and the products and then add up all the Gf for the products and then then subtract all the added reactant Gf from that. Next plug that delta G into this equation:
ΔG= -nFEocell
Where n is the moles of electrons from the equations and F is the faraday's constant = 96486 J/(V x mol of e-). This will give you the oxidation reduction potential so flip the sign on it so that we can use it in this equation:
Eocell = Eocathode -- Eoanode
Galvanic cell: Since this is a combustion reaction we know the Eocell is positive and the reaction is spontaneous. the reaction still needs inert electrodes and a salt bridge in the final galvanic cell.
Pt(s) | C3H8(g) | CO2(g) || O2(g) | H2O(g) | Pt(s)
Q20.5.28
Complexing agents can bind to metals and result in the net stabilization of the complexed species. What is the net thermodynamic stabilization energy that results from using CN− as a complexing agent for Mn3+/Mn2+?
S20.5.28
First of all the stabilizing of a species would require it to loose free energy giving it a negative ΔG which means this reaction is also spontaneous as any reaction with a negative ΔG is spontaneous. Since the reaction is spontaneous than Eocell must be positive with this equation:

